The U.S Food and Drug Administration (FDA) cleared the EverFlex Self-Expanding Peripheral Stent System and outlined unsuitable candidates for the system.
Cephasonics today announced the introduction of the cQUB-1 (cQuest Ultrasound Box-1), the first product in a new family of cQUB white-label ultrasound systems designed and manufactured by Cephasonics for purchase by companies to rebrand with their own brand name and model number.
The quality and resolution of X-ray images depends on the characteristics of the focal point, the area that is struck by electrons and from which the resulting X-rays are emitted. A new ASTM International standard will allow users to determine the effective focal spot size of an X-ray source.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
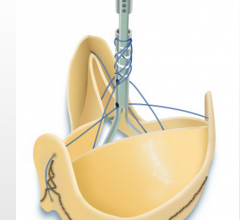
The U.S. Food and Drug Administration (FDA) approved the Sorin Freedom Solo and Solo Smart stentless heart valves from Sorin Group Canada Inc. to replace a diseased, damaged or malfunctioning aortic heart valve.
The use of stents has improved management and outcomes of coronary artery disease, and clinical trials are attempting to prove the same will be true for superficial femoral artery disease. Randomized trials have shown favorable results for self-expanding nitinol stents compared with balloon angioplasty. A new report seeks to test this treatment in a real-world population of patients enrolled in an observational registry.
Cigna Corporation has reinstated coverage of the Ambulatory Cardiac Telemetry (ACT) service and is retroactively effective as of June 15, 2014.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Lombard Medical Inc., a medical device company focused on endovascular aneurysm repair (EVAR) of abdominal aortic aneurysms (AAA), today announced that its lead product, Aorfix, an endovascular stent graft to treat AAA, has received approval from the Japanese Ministry of Health, Labour and Welfare. Commercial sales will follow reimbursement approval. Aorfix will be exclusively distributed by Medico's Hirata Inc. Japan is the world’s second-largest standalone EVAR market.
The Cardiovascular Cell Therapy Research Network (CCTRN) has selected Cytori Therapeutics Inc. to supply adipose-derived regenerative cells (ADRC) for a clinical trial aimed at evaluating the safety and feasibility of treating patients with left ventricular assist devices (LVADs). The CCTRN is supported by grants from the National Heart, Lung and Blood Institute (NHLBI), a division of the National Institutes of Health (NIH). This trial, named CELLVAD-ADRC, will explore outcomes following administration of a regenerative cell preparation from adipose tissue into the patient’s heart muscle 60 to 90 days after placement of an LVAD.
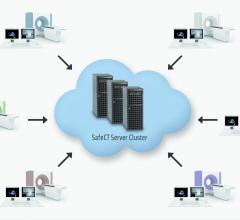
Healthcare organizations looking to implement low dose protocols for computed tomography (CT) exams on all scanners, regardless of disparate locations, can now benefit from SafeCT Enterprise from Medic Vision. The solution will be displayed at RSNA 2014.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Cardiothoracic and Vascular Surgeons became the first in Texas to implant the Solo Smart Aortic Pericardial Heart Valve. Faraz Kerendi, M.D. and Stephen J. Dewan M.D., performed the procedure at Heart Hospital of Austin.
CardioDx Inc., a molecular diagnostics company specializing in cardiovascular genomics, today announced the presentation of the study, "The Use of a Gene Expression Score Showed Clinical Utility in Evaluating African Americans Presenting with Symptoms Suggestive of Obstructive Coronary Artery Disease in a Primary Care Practice," at the 36th annual North American Meeting of the Society for Medical Decision Making, taking place from Oct. 18-22 in Miami.
A research study published in the July issue of the Journal of the American College of Cardiology reports that genetic mutations in a neuronal sodium channel gene are associated with inherited sudden cardiac death syndromes, including the Brugada syndrome. The study, performed at the Cardiac Research Institute at Masonic Medical Research Laboratory (MMRL) in Utica, N.Y., included physicians and scientists from throughout the world who referred patients with life-threatening cardiac arrhythmias to the MMRL for genetic screening.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Infinitt North America will be featuring Dose M 2.0 radiation dose tracking software at this year’s RSNA in Chicago. The new version expands the capture of dose data from multiple-modalities and can be integrated with radiology information systems (RIS), picture archive and communication systems (PACS) or electronic medical records (EMRs) to make dose information accessible as part of the patient record.
Calgary Scientific Inc. has gained U.S. Food and Drug Administration (FDA) clearance of ResolutionMD 4.0 diagnostic medical imaging software for all modalities and China Food and Drug Administration (CFDA) certification for diagnosis on mobile devices in China.
Siemens Healthcare has announced that the U.S. Food and Drug Administration (FDA) has cleared the new Prime edition of its Acuson SC2000 premium cardiovascular imaging system — a system that offers live full-volume color Doppler imaging of heart valve anatomy and blood flow using a new true volume transesophageal echo (TEE) probe.

 October 27, 2014
October 27, 2014