Edwards Lifesciences Corp. has rolled out a next-generation transfemoral delivery system for the Edwards SAPIEN ...
May 13, 2008 - Philips today announced it has reached an agreement to acquire Brazilian-based Dixtal Biomédica e Tecnologia (Dixtal), a Brazilian manufacturer of in-hospital patient monitoring systems, in a move to bolster Philips’ healthcare presence in high-growth emerging markets .
GE Healthcare launched its new mobile Vivid S5 cardiovascular ultrasound system, created for multiple care areas ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
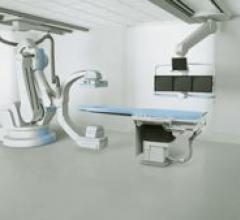
Siemens received FDA 510(k) clearance for Artis zeego, a robotic-assisted positioning capability for interventions in ...
May 13, 2008 - Edwards Lifesciences Corp. launched a next-generation transfemoral delivery system for the Edwards SAPIEN ...
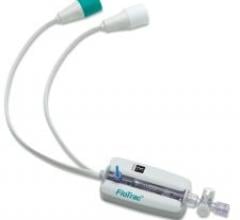
Textronics Inc. received FDA clearance to market its textile-based ECG Electrode for use in general electrocardiograph monitoring and recording procedures, offering patients an alternative to adhesive electrodes and metal wristbands.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
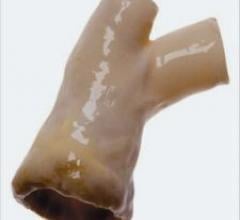
The FDA cleared CryoLife’s CryoValve SG pulmonary human heart valve processed with the company’s proprietary SynerGraft ...
May 13, 2008 – The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, said to be the world’s first high-definition CT ...
The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, said to be the world’s first high definition CT scanner that ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
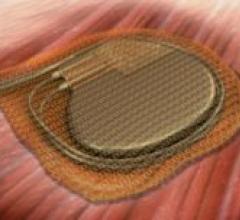
The FDA granted 510(k) clearance for the AIGISRX CRMD Anti-Bacterial Envelope by Tyrxpharma, a cardiac rhythm medical ...
May 13, 2008 - Medtronic received FDA premarket approval (PMA) for the first wave of cardiac rhythm disease management ...
Abbott Vascular’s HI-TORQUE WHISPER guidewire is designed to offer a higher level of support to facilitate device ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Edwards Lifesciences unveiled a new software upgrade that allows greater flexibility to trend and analyze the ...
May 13, 2008 – The FDA approved changes to the U.S. product label for DEFINITY Vial For (Perflutren Lipid Microsphere) ...
The FDA cleared for marketing a new automated version of diaDexus’ proprietary PLAC Test, an automated immunoassay designed to run on common existing laboratory equipment, including clinical chemistry analyzers from Hitachi, Roche/Hitachi and Olympus, expanding the number of clinical labs and physician offices that are able to offer the PLAC Test.

 May 12, 2008
May 12, 2008