Philips Healthcare recently installed an Allura Xper FD 20/20 biplane angiography X-ray system at Primary Children's Medical Center in Salt Lake City, with a National Basketball Association (NBA) theme to put children at ease prior to and during interventional radiology procedures.
April 12, 2012 - Boston Scientific Corporation announced the United States market launch of its Z Flex-270 Steerable Sheath. The device is intended for use in a wide range of electrophysiology (EP) procedures to facilitate the introduction and placement of diagnostic and therapeutic catheters within the heart. The company plans to launch the product immediately in the United States.
April 12, 2012 - Edwards Lifesciences Corp. said a U.S. Food and Drug Administration (FDA) advisory panel will review the company's request to expand the indication for the Sapien transcatheter aortic valve to high-risk surgical patients. The premarket approval (PMA) application is set to be reviewed June 13, 2012. Edwards submitted a PMA application in April 2011 based on data from the high-risk cohort (Cohort A) of the PARTNER Trial, for approval of this therapy in the treatment of patients with severe, symptomatic aortic stenosis who are at high risk for surgery.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The U.S. Food and Drug Administration (FDA) approved an expanded indication for Medtronic’s cardiac resynchronization therapy with implantable cardioverter defibrillator (CRT-D) devices. With the approval, this advanced therapy can now be used earlier, in a mildly symptomatic heart failure patient population, potentially improving survival, reducing hospitalizations and preventing disease progression.
April 12, 2012 — The Maryland General Assembly set a high bar for the nation and showed its commitment to patients by passing legislation establishing an independent review of the placement of stents in heart patients to ensure consistency with guidelines developed by the American College of Cardiology and other organizations, the Maryland Chapter of the American College of Cardiology said.
ReCor Medical disclosed updated data for the REDUCE First-In-Man clinical study of its CE-marked Paradise (percutaneous renal denervation system) ultrasound platform, which is designed to treat patients with resistant hypertension, a major risk factor for cardiovascular disease.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Accustomed to paper-based workflow processes and legacy single-modality solutions, most cardiology departments lag several years behind radiology in their adoption of cardiovascular image and information management systems (CIIMS). After providers have met the requirements for the first stages of meaningful use through their electronic medical record (EMR) implementation, the CIIMS market will likely start to benefit from IT stimulus funding starting in 2014.
Boston Scientific Corp. announced the CE mark approval and European market launch of its Ingenio and Advantio pacemakers and Invive cardiac resynchronization therapy pacemakers (CRT-P).
April 11, 2012 - The Department of Health and Human Services (HHS) announced a proposed rule that would delay, from Oct. 1, 2013 to Oct. 1, 2014, the compliance date for the International Classification of Diseases, 10th edition diagnosis and procedure codes (ICD-10).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
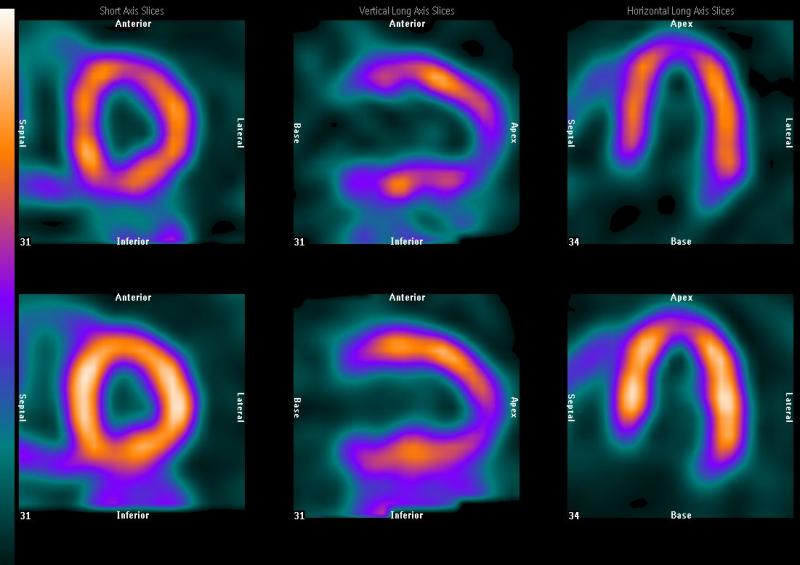
April 9, 2012 — After witnessing a downtrend for several years, revenues in the U.S. nuclear medicine and positron emission tomography (PET) imaging systems market finally pivoted in 2010. New radiotracers and technologies are expanding the clinical scope and customer base of nuclear medicine and PET imaging. As a result, declines in retrenching areas of the market are poised to be offset by strong growth in emerging end-user market segments.
April 9, 2012 — Thoratec and the U.S. Food and Drug Adminstration (FDA) announced a recent Class 1 recall of the HeartMate II left ventricular assist device (LVAD) because detachment of the bend relief from its intended position around the proximal outflow graft may allow the graft to kink or deform, resulting in reduction of blood flow. The kink also may cause pump/graft thrombosis or perforation of the outflow graft. Additionally, the metal end of the bend relief may be sharp and cause erosion and cutting of the outflow graft.
April 9, 2012 — Healthcare supply contracting company Novation released its 2012 Diagnostic Imaging Watch report. Peer reviewed by members of Novation's Diagnostic Imaging Council, the report is an overview of the latest product technology and trends for imaging subspecialties.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
April 6, 2012 — Crux Biomedical announced that a recently completed pivotal trial of its Crux Vena Cava Filter (VCF) System with bi-directional retrieval was presented at the 2012 Society of Interventional Radiology (SIR) Annual Scientific Meeting, March 24-29, in San Francisco. Vena cava filters are designed to trap blood clots that can lead to potentially fatal pulmonary embolisms (PE) among patients at risk. In the study, deployment and retrieval each were achieved with a 98 percent success rate.
April 6, 2012 — St. Jude Medical Inc. announced it has appointed Population Health Research Institute (PHRI), an academic health science research institute, to analyze data from three combined registries on the Riata ST Optim and Durata implantable cardioverter defibrillator (ICD) leads. The analysis will be entirely under the control of PHRI, who will complete a review of the performance of the leads.
April 6, 2012 — The U.S. anticoagulant market is on the verge of a major shift in clinical practice, transitioning from a market dominated by a single injectable anticoagulant to a highly competitive one dominated by first-in-class novel oral anticoagulants. Companies are vying with each other to introduce novel therapies that offer superior safety, efficacy and convenience to patients and physicians.

 April 12, 2012
April 12, 2012