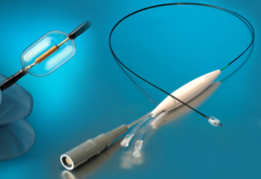
April 11, 2012 — ReCor Medical disclosed updated data for the REDUCE First-In-Man clinical study of its CE-marked Paradise (percutaneous renal denervation system) ultrasound platform, which is designed to treat patients with resistant hypertension, a major risk factor for cardiovascular disease.
Preliminary F-I-M clinical data for Paradise were reported previously at the “TRenD 2012” transcatheter renal denervation scientific meeting by cardiologist Thomas A. Mabin, M.D., Vergelegen Medi-Clinic, South Africa. The updated Paradise data show that systolic blood pressure (BP) was reduced by a statistically significant average of 36 mm Hg in eight patients at 90-day follow-up. The scientific literature demonstrates that only a 5 mm Hg reduction in BP results in a 14 percent decrease in stroke, a 9 percent decrease in heart disease and a 7 percent decrease in mortality.
“Our Paradise system?is designed to offer a minimally invasive ultrasound therapy to resistant hypertension patients to help reduce their blood pressure,” said Mano Iyer, CEO of ReCor Medical. “These updated clinical data will boost our momentum as we make ready to launch Paradise in Europe.”
Paradise includes a 6 French-compatible catheter with a cylindrical transducer that emits ultrasound energy circumferentially, allowing for a rapid and highly efficient renal denervation procedure. The advantage of Paradise is its ability to uniformly denervate all the way around the arterial wall while simultaneously cooling the endothelium, to help enable a safe, consistent and fast renal denervation procedure.
For more information: www.recormedical.com


 February 06, 2026
February 06, 2026 









