May 28, 2008 - A team of cardiologists, radiologists and front-line physicians at Intermountain Medical Center in Murray ...
May 28, 2008 - Adding noninvasive imaging to current risk-assessment protocols may identify more people who are at risk ...
May 27, 2008 - Cook Medical (Cook) has received approval from the FDA for its Zenith TX2 Thoracic TAA Endovascular Graft, marking the expansion of Cook Medical’s portfolio of devices to treat aortic diseases.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Cook Medical's Zenith TX2 Thoracic TAA Endovascular Graft is designed for thoracic endovascular aortic repair (TEVAR).
May 26, 2008 – Spectranetics introduced the new LLD EZ Lead Locking Device (LLD) for the removal of nonfunctional or ...
May 27, 2008 - Vascular Solutions will partner with Radius Medical Technologies for the U.S. launch of the MICRO Elite ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
May 27, 2008 - Angiomax (bivalirudin) significantly improved net clinical adverse events by 24 percent as well as ...
Vascular Solutions is partnering with Radius Medical Technologies to distribute in the U.S. the new MICRO Elite Snare, a device designed for the retrieval and manipulation of foreign bodies located in the coronary and peripheral cardiovascular system and the extra-cranial neurovascular anatomy.
May 22, 2008 - Nassir Marrouche, M.D., and his research team from the University of Utah School of Medicine received the ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
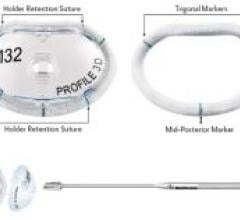
Medtronic introduced Profile 3D Annuloplasty Ring with a design based on the geometry of the saddle-shaped human mitral ...
May 22, 2008 - MIV Therapeutics Inc. has enrolled the first patients in the registration trial of its VESTAsync polymer ...
May 21, 2008 - ICU patients are nearly one-third less likely to die, on average, when treated at hospitals using the ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 21, 2008 - More than half of patients primarily receive information about device recalls from the media and not from ...
May 13, 2008 - Medtronic received FDA premarket approval (PMA) for the first wave of cardiac rhythm disease management therapies under the new Vision 3D portfolio, which will comprise a full line of implantable cardioverter-defibrillators (ICDs), cardiac resynchronization therapy-defibrillators (CRT-Ds), pacemakers and cardiac resynchronization therapy-pacemakers (CRT-Ps) to address the needs of p
May 21, 2008 - The Heart Rhythm Society and the Pediatric Congenital Electrophysiology Society (PACES) have released the ...

 May 27, 2008
May 27, 2008