Following cath lab procedures, the final step is to attain hemostasis at the arteriotomy access site. Manual compression has been the gold standard for decades, but vascular closure devices (VCDs) can speed wound closure, and hemostatic pads can help decrease clotting times. VCDs and the new dressings also accelerate patient ambulation.

Coronary chronic total occlusions (CTOs) are found in about one-third of patients who undergo angiography.(1) Most of these patients are referred to coronary artery bypass graft (CABG) surgery or medical therapy because these lesions are very challenging to treat using conventional percutaneous coronary interventional (PCI) techniques.
Warren Hospital, an acute care community hospital serving Warren and Hunterdon counties in rural New Jersey, supports 214 patient beds and 140,000 outpatient visits per year.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
In the aviation industry, changing from mechanical flight control systems to fly-by-wire electronic systems improved the capability and safety of airplanes. Like aviation, medicine has witnessed similar technological breakthroughs.

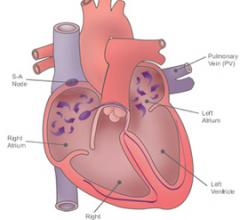
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is a major risk factor for developing emboli leading to stroke. A new approach to preventing embolisms and stroke is the use of percutaneous occlusion of the left atrial appendage (LAA).
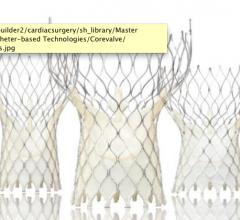
September 7, 2010 – European CE mark approval was granted for a new Medtronic CoreValve delivery system with AccuTrak Stability Layer for transcatheter aortic valve implantation (TAVI). AccuTrak allows physicians to achieve enhanced control and accuracy in the deployment of the CoreValve device.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 7, 2010 – Enrollment started in the Endovascular Atherectomy Safety and Effectiveness (EASE) study to evaluate the safety and effectiveness of the Phoenix Atherectomy catheter. The minimally invasive device treats peripheral arterial disease (PAD) in the legs. The U.S.
September 7, 2010 – New analyses of subgroups from the SORT OUT III study provide detail on longer-term follow-up safety and efficacy outcomes in diabetics and patients with acute coronary syndrome and multiple lesions.Three new analyses of subgroups were presented at the European Society of Cardiology (ESC) meeting last week in Stockholm.
September 7, 2010 - With increasing rates of patients with atrial fibrillation program suffering from severe strokes, medical associations fear an epidemic. New developments in minimally invasive therapy of atrial fibrillation are booming and will be evident at MEDICA 2010, World Forum for Medicine - International Trade Fair and Congress, to be held from Nov.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 2, 2010 - The STAR-heart study, which was presented at the European Society of Cardiology 2010 Congress in August 2010, reported that the intracoronary injection of autologous stem cells derived from bone marrow is associated with improved hemodynamics and long term survival in the treatment of chronic heart failure.
September 2, 2010 - A single-patient disposable blood pressure cuff that travels with the patient throughout her hospital stay is reducing the risk of spreading infectious disease and decreasing overall costs.
September 2, 2010 – The Smithsonian's National Museum of American History recently accepted the donation of a prototype medical emergency crash cart, referred to as MAX. It accepted the donation from ECRI Institute, an independent nonprofit that researches the best approaches to improving patient care.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
September 2, 2010 – New research released this week is the first to find both anatomical and procedural considerations that can lead to the creation of esophageal ulcerations (ESUL) after radiofrequency ablation of atrial fibrillation (AF).
September 2, 2010 – Artificial heart maker SynCardia Systems said it plans to put the company up for sale some time in the next two years.
September 2, 2010 - Women and men with a 10-year cardiovascular disease risk of 5 percent or more and normal cholesterol levels but high levels of hsCRP, a protein associated with inflammation, could reduce their risk substantially with statin therapy, according to new research.

 September 07, 2010
September 07, 2010