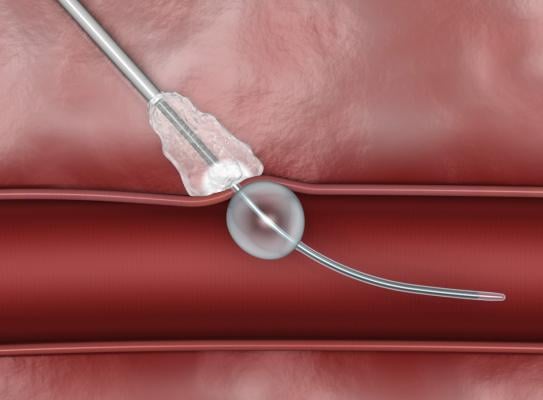
The bioresorbable Cordis MynxGrip vascular closure device seals arteriotomies without use of permanent hardware.
While manual compression remains the gold standard for hemostasis of catheterization vascular access site arteriotomies, the current cost-conscious era of making healthcare more cost-effective has renewed interest in technologies to speed vascular closure.
While using these devices may increase procedural costs, users of these technologies say there is a lot of savings downstream by achieving faster hemostasis. These benefits include reduced bleeding complications, a reduction in post-procedural nursing care time required, increased patient throughput, increased patient satisfaction and faster patient ambulation. Getting patients up and around more quickly also aids hospitals that have same-day percutaneous coronary intervention (PCI) programs.
These devices are divided into three categories:
• Vascular closure devices that immediately seal the vessel using mechanical means;
• Compression devices that strap onto the patient to put pressure on the arteriotomy site; and
• Hemostatic pads that use materials to speed up the clotting cascade to reduce time to hemostasis.
Often hemostatic pads and manual or device-assisted compression are used in combination.
Changes in the Vascular Closure Market
Abbott Vascular purchased St. Jude Medical in early 2016. This included duplicate vascular closure products in its new, combined product portfolio. So, in October 2016, Abbott sold off some of its vascular closure products to Terumo Corp. The $1.12 billion price tag included St. Jude Medical's Angio-Seal and Femoseal vascular closure products and Abbott's Vado steerable sheath used to navigate electrophysiology ablation catheters.
Abbott retained its other vascular closure products, which include the Perclose ProGlide Suture-Mediated Closure System, StarClose SE Vascular Closure System and Prostar XL Percutaneous Vascular Surgical System. The Angio-Seal product lines offer healthcare providers an alternative to manual compression for sealing puncture sites on patients who have undergone a catheterization procedure.
The newest entry into the U.S. vascular closure market is Vasorum Ltd. with its Celt ACD system. It gained U.S. Food and Drug Administration (FDA) clearance in July 2016.
It can immediately seal 5, 6 and 7 French punctures, and was designed to be more clinically versatile than other devices. This includes allowing multiple re-stick procedures and addressing a broader range of clinical situations and patient
anatomies. The company said the device closes multiple resticks in calcified vessels. The company is also developing a large-bore device to close 12-14 French punctures.
“The Celt ACD range of closure devices are addressing the clear need for quicker and more efficient methods of increasing patient throughput in healthcare facilities, and they provide doctors and patients with a solution which can efficiently manage arterial closure following vascular procedures,” said Shing-Chiu Wong, M.D., director of cardiac catheterization at the NewYork-Presbyterian/Weill Cornell Medical Center, who has carried out the first commercial cases in the U.S.
Bioresorbable Vascular Closure Technologies
A big issue for opponents of vascular closure devices is that many of these devices leave behind metal hardware that become a permanent part of the vessel wall. They argue these components can lead to the development of scar tissue or cause problems for future vessel access.
One of the first successful bioresorbable technologies was the MynxGrip vascular closure device from Cordis (a division of Cardinal Health). It uses a polyethylene glycol (PEG) polymer as sealant that adheres to the contours of the vessel wall, providing active closure of the arteriotomy. The sealant fully resorbs within 30 days.
In 2016, the FDA granted market clearance to Rex Medical’s bioresorbable Closer Vascular Sealing System (VSS) to achieve rapid hemostasis of femoral artery catheterizations. It consists of an insertion sheath, dilator and an implant contained in a delivery system. It uses a patch placed against the artery wall and is secured on the outside of the vessel by an attached suture connected to two bioresorbable spheres. The suture brings the patch and spheres together. The patch and spheres are made of polylactide-co-glycolide acid co-polymer. The suture is made of polydiaxanone. The components of the device dissolve inside the body after the vessel heals.
Radial Access Spurs Growth in Wrist Compression Devices
The use of inexpensive wristband devices for radial artery access site compression is not new, but their usage has greatly increased over the past few years. Radial access usage in the United States has steadily increased to about 30 percent of PCI cases, according to Sunil Rao, M.D., associate professor of medicine at Duke University Medical Center, who tracks radial usage statistics from the ACC-NCDR registry. The U.S. usage was only around 10-15 percent in 2010.
The radial artery is easier to close than femoral access sites and has fewer bleeding complications. Additionally, patients with a compression wristband can ambulate immediately and walk out of the cath lab, go to the bathroom on their own, reducing post-procedural nursing care and greatly enhancing patient comfort.
This rapid growth in radial has seen new vendors entering this space. Among these is Medtronic, which launched an entire radial access product portfolio in February 2017. This included the TRAcelet compression device, which simplifies pressure reduction post-transradial catheterization by facilitating access site hemostasis while maintaining radial artery patency.
DAIC’s hemostasis management comparison chart is divided into three sections. The interest in radial is reflected in the chart section for compression devices, which had the most submissions from vendors, over vascular closure devices and hemostatic pads.
New Large-bore Vascular Closure Devices
There has been a rapid increase in the number of interventional procedures requiring larger devices, which require larger-bore vascular access arteriotomies. Many devices used for transcatheter aortic valve implantation (TAVI), endovascular aneurysm repair (EVAR), percutaneous ventricular assist device (VAD) implantation and balloon aortic valvuloplasty (BAV) require access between 10-25 French, which can be difficult to seal through conventional means. Large-bore femoral access sites have been associated with significant morbidity, including long times to achieve hemostasis, extended procedure time, need for a vascular surgeon in the catheterization lab if surgical cut downs are used, delayed ambulation, higher complication rates and higher total cost of care. In response, a few vendors have developed hemostasis devices to address this growing need.
In December 2017, Essential Medical said it completed enrollment for its U.S. pivotal investigational device exemption (IDE) trial of the Manta large-bore vascular closure device. The study enrolled 341 patients at 21 different sites in 10 months. The company expects to file a premarket approval (PMA) submission with the FDA by the end of the first quarter of 2018. Manta has European CE mark to close punctures ranging from 10-25 French.
The Manta device uses a collagen implant secured by an intraluminal anchor designed to better seal large-bore cannulation. The company said it can seal high-pressure vessels in less than 60 seconds.
"Beautiful device once you learn the nuances. John Webb and I deployed over 30 Manta devices at Vancouver General Hospital and St. Paul's Hospital and we are very encouraged by the results,” said David Wood, M.D., co-principal investigator of the U.S. MANTA trial and a founding member of the Centre for Heart Valve Innovation in Vancouver, Canada. “It will be tough going back to our standard of care while we await commercial approval."
Another company that has developed a large-bore closure device is Vivasure Medical. Its PerQseal is a fully bioresorbable device designed to seal TAVR and EVAR access sites. It received CE mark in January 2016. It uses a polymer implant that deploys inside the vessel and hugs the vessel wall to act as a support scaffold. The abluminal surface of the patch is textured, to promote adherence of the patch to the vessel wall. A portion of the scaffold extends through the arteriotomy, and includes a locator which helps maintain the implant in position. After deployment, the implant is rapidly endothelialized and is fully absorbed.
Vivasure announced it raised $18.3 million in funding in the fall of 2016 to support European commercialization of the PerQseal and to cover the expenses of an FDA regulatory study.
Vascular Closure Comparison Charts
DAIC has three charts involving hemostasis management. Access to specifications requires a login, but it is free and only takes a minute to complete.
• Hemostasis Management Compression Devices
• Hemostasis Management Hemostatic Pads
• Hemostasis Management: Vascular Closure Devices



 November 14, 2025
November 14, 2025 









