
Navin Kapur, M.D., FAHA, FACC, FSCAI, executive director, The CardioVascular Center for Research and Innovation (CVCRI), explains the concpet of the Door-to-Unloading (DTU) Trial he is heading, one of several innovations at Tufts Medical Center in Boston. Kapur is also director, Acute Mechanical Circulatory Support Program; director, interventional research laboratories; director of Cardiac Biology Research Center, Molecular Cardiology Research Institute (MCRI), Tufts Medical Center. Photo by Dave Fornell
The cardiology program at Tufts Medical Center in Boston is internationally recognized for being on the forefront of clinical research, and also for the use of new devices and techniques to improve care and advance scientific understanding. DAIC had the opportunity to spend a couple days at Tufts in December 2019 to document various aspects of the program and what makes it innovative.
Many of its physicians are thought leaders and can be found as regular speakers at several cardiology conferences. They are supported by researchers and resources from the Tufts Molecular Cardiology Research Institute (MCRI), the Tufts Cardiovascular Center for Research and Innovation (CVCRI) and the Tufts preclinical Interventional Research Laboratories (SIRL). All of these entities are located on site and enable close, real-time collaboration with the practicing cardiologists. These resources have enabled Tufts to become a clinical partner with numerous cardiac device vendors to both preclinical and clinical studies of new technologies.
"The unique aspect of the Tufts program and why it has been very successful the past couple decades is that housed within the medical center campus has been one of the most phenomenal cardiovascular centers, a top 10 program for heart transplants, top 10 program for LVADs, and a very highly integrated network of hospitals for cariogenic shock and acute mechanical support that we have built over the past 10-15 years," explained Navin Kapur, M.D., FAHA, FACC, FSCAI, executive director, The CardioVascular Center for Research and Innovation (CVCRI), director, Acute Mechanical Circulatory Support Program; director, interventional research laboratories; director of Cardiac Biology Research Center, Molecular Cardiology Research Institute (MCRI), Tufts Medical Center. "Coupled with that we have on site the Molecular Cardiology Research Institute, which is 14 stories of fundamental discovery biology, and it is about 100 yards from the cath lab. And the third component we have is a 30,000 square foot research laboratory with three hybrid ORs that allows us to take everything we do very well clinically and and improve upon it in a research laboratory. And when we have questions about fundamental mechanisms, we can bring in the Molecular Institute. And then all of that work goes right back into the cath lab and the clinical program."
Kapur is the maestro of these programs and has been at the forefront of Tufts' cardiovascular research. With these tools at his disposal he and his team have pioneered in several areas of cardiovascular medicine.
Door-to-Unloading (DTU) Trial May Change STEMI Care
Kapur and Tufts are currently leading a pivotal clinical trial that is testing a new method that could be a paradigm shift in how to care for patients with ST-elevated myocardial infarction (STEMI). The Door-to-Unloading (DTU) Trial uses the catheter-based Impella hemodynamic support device to unload the heart 30 minutes prior to percutaneous coronary intervention (PCI). The concept is to initiate early hemodynamic support prior to PCI, which pre-clinical and pilot studies showed reduced or eliminated the ischemia and limited myocardial damage. It also appears to help reduce the no-reflow phenomenon, reperfusion injury that occurs in some heart attack patients who are revascularized.
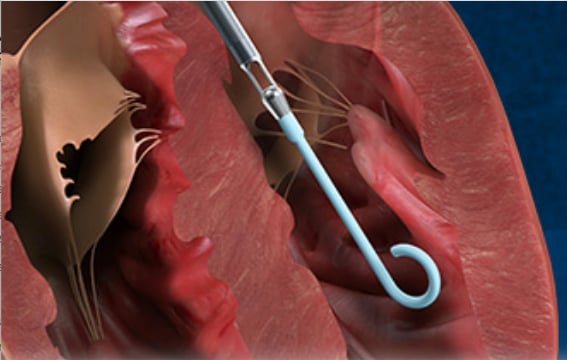 "This is a landmark trial that has already begun, and it's built upon decades of science. The goal is to change the trajectory of patients who are presenting with STEMI. We know we have done a very good job of reducing in-hospital mortality, but we are also seeing over the past 20 years a substantial number of patients going on to develop heart failure. And a major determinant is the amount of muscle damage," Kapur explained.
"This is a landmark trial that has already begun, and it's built upon decades of science. The goal is to change the trajectory of patients who are presenting with STEMI. We know we have done a very good job of reducing in-hospital mortality, but we are also seeing over the past 20 years a substantial number of patients going on to develop heart failure. And a major determinant is the amount of muscle damage," Kapur explained.
He said some studies have shown if you can reduce oxygen demand prior to PCI, it results in better outcomes and much less myocardial damage than just going directly in and revascularizing the artery.
"Trying to treat reperfusion injury has been a Holy Grail for a long time in cardiovascular medicine," Kapur said.
He said drugs have been developed in attempts to treat the phenomenon, but the ability to test them has been hampered because of the mandate to revascularize patients as soon as possible to reduce door-to-balloon times.
"We are approaching this as a method to reduce repercussion injury by first unloading the heart, resting the heart and making the heart more receptive reperfusion so there would be less injury. But what we have learned in our studies, is that the moment a patient is unloaded, you are now beginning to balance that supply/demand mismatch, and as a result the myocardium may no longer be ischemic. So the current trial will compare door-to-balloon time to door-to-unload. This will compare two anti-ischemic approaches of reducing oxygen demand to restoring oxygen supply, but recognizing by restoring blood supply there might be a cost involved."
Kapur admits the idea of putting in an Impella and waiting 30 minutes to perform PCI may sound crazy and completely counterintuitive to the current standard of care.
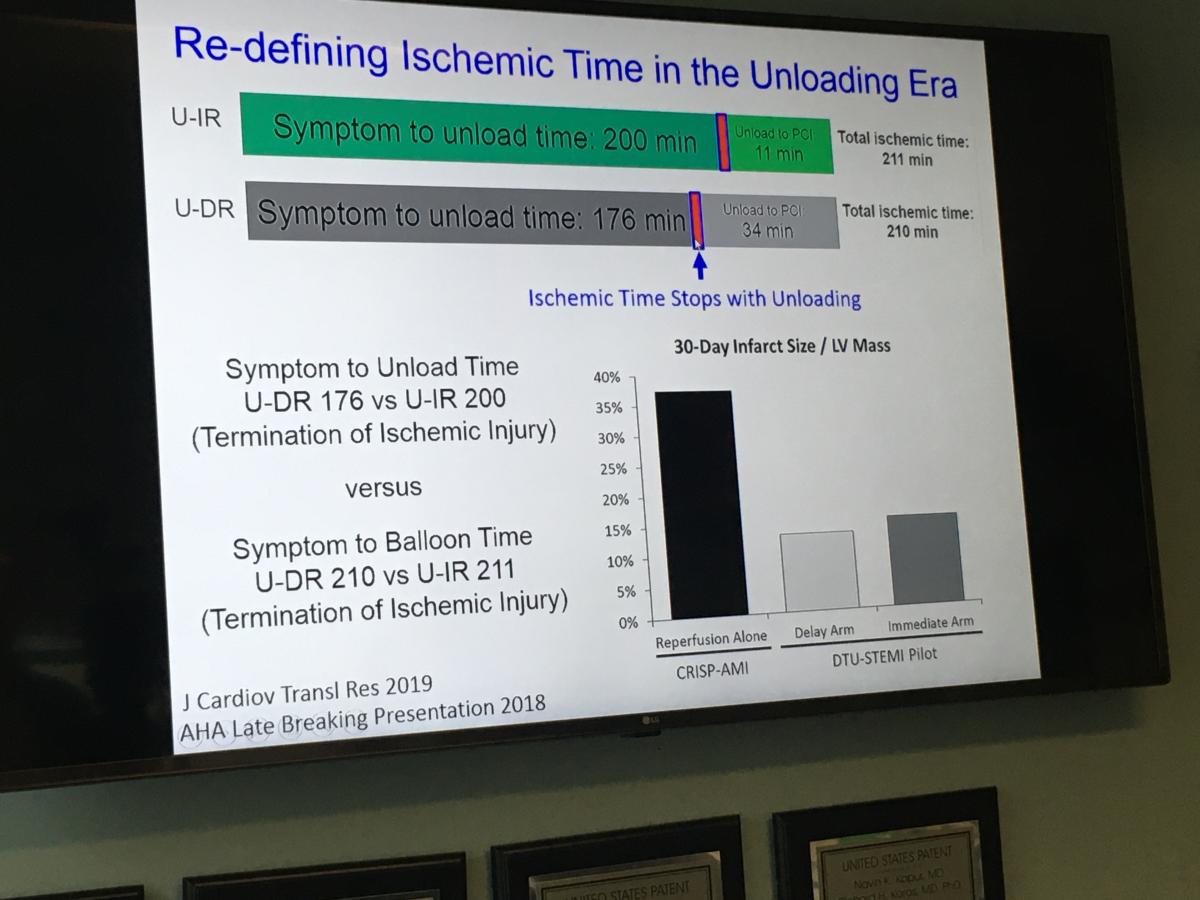 "I think the main concept people are still wrapping their heads around is this idea of waiting 30 minutes," Kapur explained. "But in the DTU pilot study we learned it is safe to do an Impella implant in the middle of a STEMI, and we learned that operators can wait 30 minutes and it was safe and it did not increase infarct size or cause any hemodynamic compromise."
"I think the main concept people are still wrapping their heads around is this idea of waiting 30 minutes," Kapur explained. "But in the DTU pilot study we learned it is safe to do an Impella implant in the middle of a STEMI, and we learned that operators can wait 30 minutes and it was safe and it did not increase infarct size or cause any hemodynamic compromise."
However, the biggest takeaway from the pilot study was that patients with the largest myocardial infarctions, despite having a longer exposure to an occluded left anterior descending (LAD) artery, those patients actually had smaller infarcts than patients who underwent immediately reperfusion.
The Latest Research on Cardiogenic Shock
Tufts heads up the Cardiogenic Shock Working Group (CSWG), which has created a patient registry that includes 15-20 centers in the United States and data on more than 2,000 patients who suffered shock either from heart failure or acute myocardial infarction (MI). The registry also includes detailed hemodynamic data on more than 1,100 patients. The registry includes all types of mechanical circulatory support devices used, from intra-aortic balloon pumps (IABP), incrementally higher levels of percutaneous Impella pump, TandemHeart and extracorporeal membrane oxygenation (ECMO).
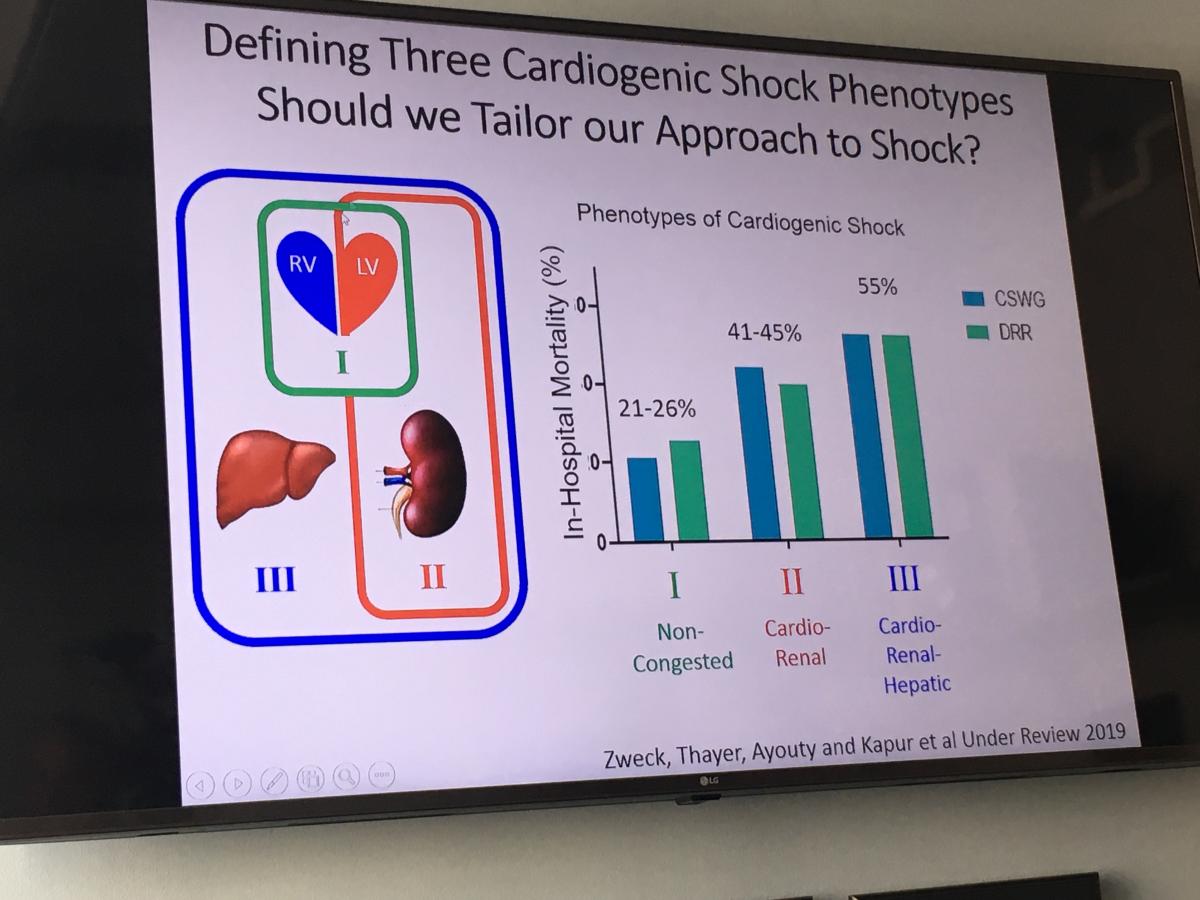 "We manage a lot of cardiogenic shock at Tufts Medical Center and we have experienced a lot of the frustration, primarily because we have not been able to move the dial on improving outcomes," Kapur said. "What the Cariogenic Shock Working Group allows us to do is collect a lot of information in a very short amount of time and generate a substantial amount of power in terms of numbers of patients in the study."
"We manage a lot of cardiogenic shock at Tufts Medical Center and we have experienced a lot of the frustration, primarily because we have not been able to move the dial on improving outcomes," Kapur said. "What the Cariogenic Shock Working Group allows us to do is collect a lot of information in a very short amount of time and generate a substantial amount of power in terms of numbers of patients in the study."
He said the hemodynamic data on patients from PA catheters has helped a lot in moving forward the understanding of shock. Research using this data last year showed three types of phenotype profiles in cardiogenic shock patients, including non-congested, cardio-renal and cardio-renal-hepatic. Kapur also said there is evidence that there is venous involvement that needs to be considered in future research.
The large amount data and variables collected by the CSWG prompted the Tufts team to develop an artificial intelligence (AI) algorithm to sift through it and identify any patterns. Kapur said this technology helped identify the three types of shock.
Hear more on the CSWG in the VIDEO: New Research in Cardiogenic Shock at Tufts Medical Center.
Tufts Spearheads Innovation With its Preclinical Cath Lab
Tufts has developed several new cardiovascular technologies from its own physicians, and is a beta test site for new technologies from numerous vendors. This includes a lot of preclinical testing of hemodynamic support systems. Tufts is one of the few cardiology centers that has both clinical and pre-clinical programs in the same location, which greatly enhances communication between the cardiologists and their pre-clinical colleagues and speeds up the process to modifying and testing new devices.
 "We have a lot of physicians who have great ideas, or they see something in the Cath lab that they are curious about, and they are able to come down here and be able to work their idea through," explained Lara Reyelt, veterinary technician and preclinical surgeon at the Interventional Research Laboratories (SIRL) at Tufts Medical Center. "Dr. Kapur was able to bench top an idea, and he actually wrote it down on a napkin and came into the lab and we played around with it for a while and we came up with an SVC occlusion catheter to treat acute decompensated heart failure, which was taken to the FDA. I was able to go up to the cath lab and observe for myself the first in human procedure with the device."
"We have a lot of physicians who have great ideas, or they see something in the Cath lab that they are curious about, and they are able to come down here and be able to work their idea through," explained Lara Reyelt, veterinary technician and preclinical surgeon at the Interventional Research Laboratories (SIRL) at Tufts Medical Center. "Dr. Kapur was able to bench top an idea, and he actually wrote it down on a napkin and came into the lab and we played around with it for a while and we came up with an SVC occlusion catheter to treat acute decompensated heart failure, which was taken to the FDA. I was able to go up to the cath lab and observe for myself the first in human procedure with the device."
Hear more about this technology is the VIDEO: Occluding the SVC as a Reset Button in Heart Failure — Interview with Navin Kapur, M.D.
She said her lab also supports multiple companies testing their devices, and look at not just how these support the heart, but how it supports the rest of the body.
"It can be a little scary when you have a brand new device coming through here and it is pretty much a thought from paper and it is brought through the engineering process, and then they have the owner of the company along with a lot of technicians to support the product. We deliver it for the first time and all hold our breath and see how well it will work. Sometimes it works fantastic, other times we have to go right back to the drawing board," Reyelt said.
The lab also pioneered a novel approach now being used in the FDA DTU Trial to treat STEMI heart attacks with Impella hemodynamic support first, followed by 30 minutes of hemodynamic support prior to revascularizing the patient with percutaneous coronary intervention (PCI). The lab also performed preliminary work with the HeartMate PHP System to determine protocols for the now ongoing SHIELD II clinical trial. The lab also was used to test several new device technologies prior clinical trials, including the Impella 5.5 device.
Reyelt said another advantage of being inside a full serve hospital is access to advanced imaging such as MRI and CT.
Some industry partners include Abiomed, Thoratec, TandemHeart, Maquet, HeartWare, Abbott and Boston Scientific.
Watch the VIDEO: Tufts Medical Center Spearheads Innovation With its Preclinical Cath Lab, interview with Lara Reyelt, veterinary technician and preclinical surgeon.
Overview of the Structural Heart Program at Tufts Medical Center
The Tufts structural heart program uses a variety of transcatheter interventional devices, including the Edwards Lifesciences Sapien transcatheter aortic valve replacement (TAVR) system, Abbott MitraClip to repair mitral and tricuspid valves, occluders to seal congenital holes in the heart, PFO closure to prevent cryptogenic stroke, alcohol septal ablation to treat hypertrophic cardiomyopathy, and the Boston Scientific Watchman device to close the left atrial appendage (LAA) in atrial fibrillation patients.
 Tufts began using TAVR immediately after FDA clearance in 2012. In August 2019, the FDA cleared TAVR for use in low-risk patients, effectively opening TAVR use for all patients that meet certain anatomical requirements. This has driven a 20-30 percent growth in Tufts TAVR program since last year.
Tufts began using TAVR immediately after FDA clearance in 2012. In August 2019, the FDA cleared TAVR for use in low-risk patients, effectively opening TAVR use for all patients that meet certain anatomical requirements. This has driven a 20-30 percent growth in Tufts TAVR program since last year.
"When it was originally approved, it was only for the highest risk surgical patients. But it has really changed over the past eight years. The randomized controlled trials show efficacy in pretty much every patient with aortic stenosis, including the very high risk patients to the vary low risk patients," explained Andrew Weintraub, M.D., FACC, associate director of the Interventional Cardiology and Vascular Center, medical director of the Vascular and Structural Heart Center.
Weintraub said the Tufts TAVR heart team sees transformoral access as the best route with the best outcomes, but also is proficient in several alternative access routes in about 5 percent of patients. These are to enable more patients to be treated using this minimally invasive procedure. They originally used transapical access, but they now primarily use axillary artery access.
"What we found in our program is that axillary access can be done as quickly as transfemoral access with similar low complication rates and very short lengths of stay," Weintraub said.
Each year the Tufts structural heart program performs about 170 TAVR procedures, and Weintraub expects the current rate of growth to soon put the program over 200 patients a year. Tufts also performs about 50 MitraClip procedures, 30 LAA occlusions, and about 35 septal ablations per year.
VIDEO: Overview of the TAVR Program at Tufts Medical Center — Interview with Andrew Weintraub, M.D.
VIDEO: Overview of the Structural Heart Program at Tufts Medical Center — Interview with Charles D. Resor, M.D.
Use of a Temporary Pacing Lead in TAVR
Implantation of TAVR valves can cause pressure from the valve pressing against the septal wall of the heart, causing conduction delays. These delays do not necessarily mean the patient needs a permanent pacemaker, but the only option has been to use temporary pacemaker leads that require the patient to be in the ICU because the leads are not actively fixed. Hospitals have been looking for a way to safely allow time to evaluate a patient after the procedure.
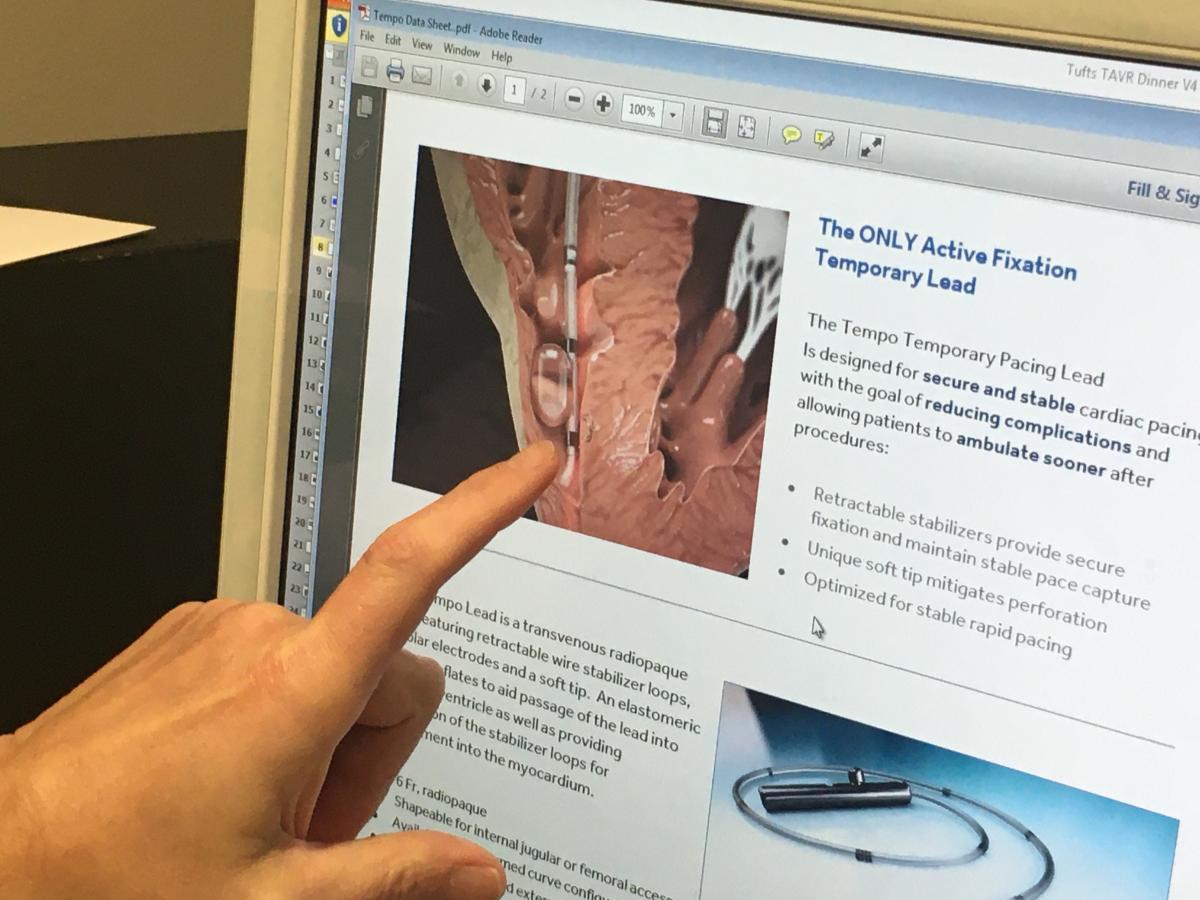 "At Tufts we have a pacemaker rate of about 9-10 percent in TAVR patients, but we have recognized that the implantation of the heart valve might cause conduction delays that do not necessarily require an urgent placement of a pacemaker," Weintraub said. "About 20 percent of our patients have conduction delays and it is these patients were grappling with."
"At Tufts we have a pacemaker rate of about 9-10 percent in TAVR patients, but we have recognized that the implantation of the heart valve might cause conduction delays that do not necessarily require an urgent placement of a pacemaker," Weintraub said. "About 20 percent of our patients have conduction delays and it is these patients were grappling with."
Tufts uses temporary pacing leads, a small catheter with two electrodes, placed in the right ventricle of the heart through a vein in the groin or neck. The lead is then connected to an external pacemaker allowing a physician to monitor and control a patient’s heart rate for up to several days. The BioTrace Medical Tempo Lead incorporates a novel active fixation mechanism, bipolar electrodes and a soft tip. Stabilizers provide secure fixation and maintain stable pace capture. An elastomeric balloon may be inflated to aid passage of the lead through the venous vasculature and into the right ventricle, and aids in wall apposition during deployment of the stabilizers. This design helps secure and stabilize the cardiac pacing lead with the goal of reducing complications and allowing patients to ambulate sooner after procedures.
"It works well. It is very stable and it actually has allowed our patients to go from the hybrid room to a post-anthesis unit and then up to the floor," Weintraub said.
The device allows 24-48 hours of evaluation by an electrophysiologist to determine if the patient needs a permanent pacemaker or not.
Watch the VIDEO: Use of a Temporary Pacing Lead in TAVR, interview with Andrew Weintraub, M.D.
Septal Ablation to Treat Hypertrophic Cardiomyopathy
The Tufts Medical Center operates the largest hypertrophic cardiomyopathy (HCM) program in New England. Each year, they perform about 35 septal ablations to treat medication-refractory HCM using a heart team approach. The team determines which HCM patients with left-ventricular tract obstruction are best served by surgical septal myectomy or alcohol septal ablation. Other HCM therapies include implantable cardioverter defibrillators (ICDs), ablation for recurrent atrial fibrillation and heart transplants.
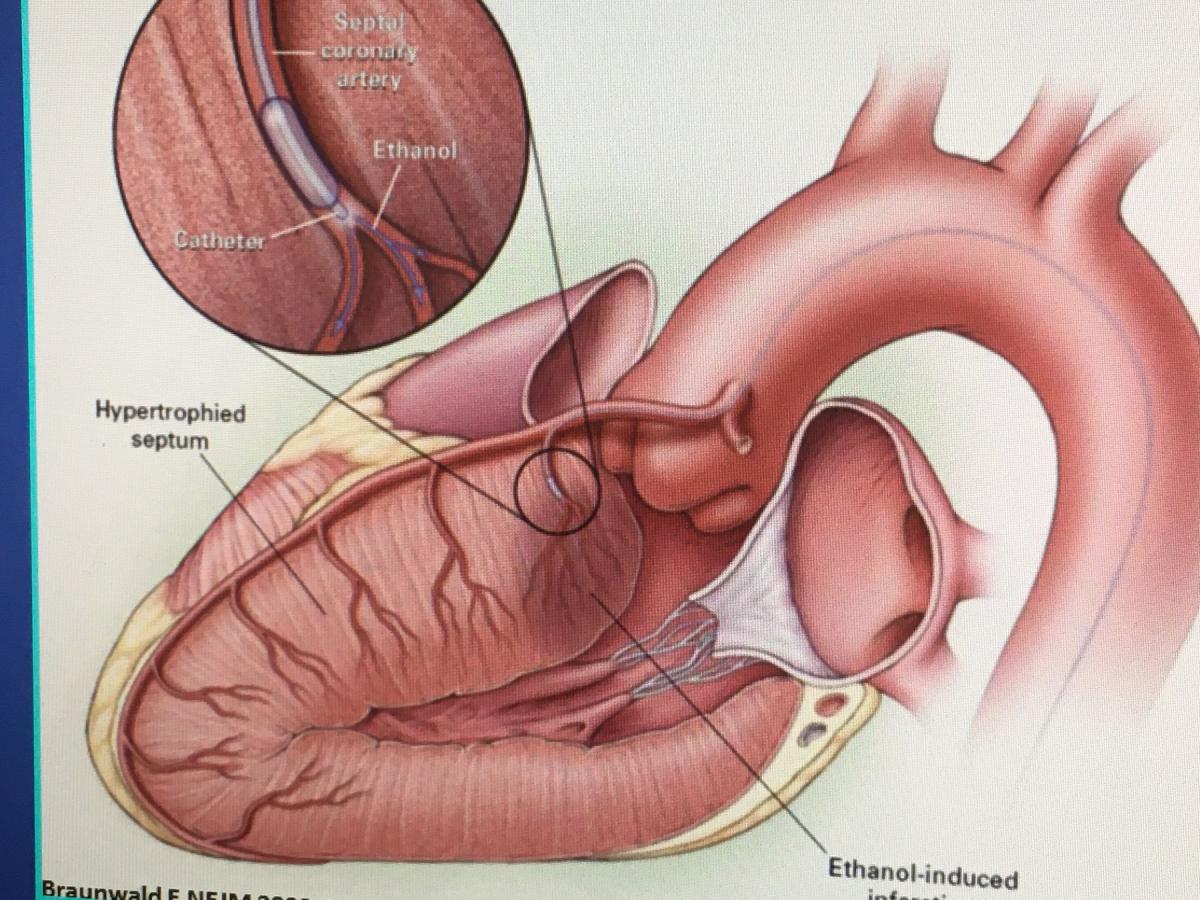 Alcohol septal ablation is used for patients who are generally not ideal candidates for the surgical myectomy operation. This procedure takes place in the catheterization laboratory without general anesthesia, and mimics the beneficial effects of surgery, explained Carey Kimmelstiel, M.D., FACP, FACC, director, cardiac catheterization laboratory, director, interventional cardiology, Tufts Medical Center, and professor of medicine at Tufts University School of Medicine. He said Tufts has conducted studies on the procedure to identify the best patient selection and techniques.
Alcohol septal ablation is used for patients who are generally not ideal candidates for the surgical myectomy operation. This procedure takes place in the catheterization laboratory without general anesthesia, and mimics the beneficial effects of surgery, explained Carey Kimmelstiel, M.D., FACP, FACC, director, cardiac catheterization laboratory, director, interventional cardiology, Tufts Medical Center, and professor of medicine at Tufts University School of Medicine. He said Tufts has conducted studies on the procedure to identify the best patient selection and techniques.
Learn more in the VIDEO: Septal Ablation to Treat Hypertrophic Cardiomyopathy — Interview with Carey Kimmelstiel, M.D.
Transcatheter PFO Closure to Prevent Stroke and Migraines
Another area of structural heart interventions performed at Tufts is transcatheter patent foramen ovale (PFO) closure. Tufts was the lead enrollment site in the Gore and Amplatzer PFO closure device trials. Cardiology works very closely with neurology to select patients who might benefits from PFO closure to help prevent cryptogenic stroke and or migraine headaches, which is the main reason why Tufts lead enrollments in the trials.
"We have collaborated very closely with neurology and have been very successful in enrolling patients," said Kimmelstiel, who was the top implanter in RESPECT Trial, and one of the lead implanters in the REDUCE Trial.
Watch the VIDEO: Transcatheter PFO Closure to Prevent Stroke and Migraines — Interview with Carey Kimmelstiel, M.D.
Using the Heart Team Approach in Heart Failure
Tufts Medical Center in Boston has created a heart failure team to better care for patients. It consists of various stakeholders, including interventional cardiology, advanced heart failure, cardiothoracic surgery, electrophysiology, cardiac imaging and critical care specialists. The advanced heart failure board meetings to review patient cases for possible heart transplants will often have 30-40 people present. Tufts also started an interventional heart failure fellowship program to better train interventional cardiologists to work on these types of teams and perform more advanced procedures.
"The idea is that the devices used to support these advanced heart failure patients, either acute devices or chronic durable devices, and the patients have become so complicated," said James Udelson, M.D., chief of the division of cardiology at Tufts Medical Center, Boston. "It now takes expertise from both the interventional angle for the implant and how to manage the device, taking out the device, and then the heart failure component for how to manage the entire patient with the device. So people who are highly trained in both of those aspects are really highly valuable to a heart failure program with a lot of complex cases."
The Tufts heart failure program offers various levels of hemodynamic support, up to left ventricular assist devices (LVAD) and heart transplants. The center is also using several cutting-edge device technologies, including an intra-atrial shunt device and controlling baroreceptors with a pacemaker-like pulse generator device. Tufts is also using devices in clinical trials, including a short-term aortic pump to augment blood flow, and balloon occlusion of the superior venacava (SVC) to mitigate some heart failure symptoms.
A key component is the heavy involvement of intensivists working directly with cardiologists to care for HF patients. Udelson said this is important, because cardiac intensive care units (CCU) are no longer just for patients in shock or recovering from heart attacks, they include patients on hemodynamic mechanical support and multi-organ failure. Using the team approach allows experts in several areas to offer suggestions for what they think the best treatment options are for the patients. The team also can better coordinate hand-offs of care and ensure there is patient follow-up.
 "We found it very helpful to integrate all the team members, including interventional cardiologists, heart failure specialists, the cardiac surgeon, cardiac anesthesiologist and the intensivist, as well as the nursing staff " explained Haval Chweich, M.D., medical director of the cardiac critical care unit (CCU) at Tufts Medical Center, and assistant professor at Tufts University School of Medicine. He said the nursing staff plays a big role since they often have the most contact with the patient and are often are the first clinicians on the team too see worsening conditions and alert the physicians.
"We found it very helpful to integrate all the team members, including interventional cardiologists, heart failure specialists, the cardiac surgeon, cardiac anesthesiologist and the intensivist, as well as the nursing staff " explained Haval Chweich, M.D., medical director of the cardiac critical care unit (CCU) at Tufts Medical Center, and assistant professor at Tufts University School of Medicine. He said the nursing staff plays a big role since they often have the most contact with the patient and are often are the first clinicians on the team too see worsening conditions and alert the physicians.
VIDEO: Developing a Heart Failure Care Team — Interview with James Udelson, M.D.
VIDEO: The Role of Intensivists on the Cardiac Critical Care Team — Interview with Haval Chweich, M.D.
Optimizing Platelet Inhibition in the Cath Lab
Tufts interventional cardiologists have access to researchers and resources of Tufts Molecular Cardiology Research Institute onsite. This has led to a lot of studies on the of optimization of GP IIb IIIa inhibitors and changing protocols for tirofiban and the use of bivalirudin, Kimmelstiel said.
"We went on to show that trofiban was really woefully under dosed at the prior 10 microgram per kilogram bolus dose. And we showed there was ineffective platelet inhibition at that approved dose at that time," Kimmelstiel explained. "We also documented the half-life of bivalirudin and, for the first time in a really good way, the antiplatelet effects of bivalirudin in showing it prevented the cleavage of PAR1 receptor."
Watch the VIDEO: Optimizing Platelet Inhibition in the Cath Lab at Tufts Medical Center, interview with Carey Kimmelstiel, M.D.
The Interventional Labs at Tufts Medical Center
 Tufts interventional cardiology program has three cath labs, one of which is a fully licensed hybrid operating room (OR). The hybrid lab was completed in 2018. Tufts interventionalists and surgeons use the room to perform some of the most advanced therapeutic procedures, including transcatheter aortic valve replacement (TAVR), MitraClip mitral valve leaflet repairs and acute mechanical circulatory support procedures.
Tufts interventional cardiology program has three cath labs, one of which is a fully licensed hybrid operating room (OR). The hybrid lab was completed in 2018. Tufts interventionalists and surgeons use the room to perform some of the most advanced therapeutic procedures, including transcatheter aortic valve replacement (TAVR), MitraClip mitral valve leaflet repairs and acute mechanical circulatory support procedures.
The room is centered on a GE Healthcare Discovery IGS angiography system that uses a robotic, laser-guided C-arm gantry that can roll around the room and is not mounted to the floor or ceiling.
"We chose that system for its versatility and for the fact that it was going to be in a hybrid OR," said Richard Botto, CVT, RCSA, chief cardiovascular technologist, division of cardiology, cardiac cath lab. "If we needed to go to an open surgical procedure for a closed procure, we can get the gantry completely out of the way and have 360 degree access to the patient. In the past when we had to covert to an open procedure, we still had the C-arm behind us and it got in the way."
Two babies also were delivered in this room due to extremely high-risk pregnancies involving severe pulmonary hypertension. Tufts is the only facility in Boston to have a fully functional operating room geographically located within the cardiac catheterization laboratory.
Watch the VIDEO: Overview of the Interventional Labs at Tufts Medical Center, an interview with Richard Botto, CVT, RCSA
Use of FFR-CT to Non-invasively Evaluate Coronary Lesion Severity
Tufts was the first hospital in Boston to adopt a new cardiac computed tomography (CT) technology that noninvasively uses the CT images to calculate the fractional flow reserve (FFR) values in the entire coronary artery tree. This FFR-CT technology can be used to determine if a coronary lesion is flow limiting and requires revascularization and can do so with the same accuracy as a catheter-based FFR measurement in the cath lab.
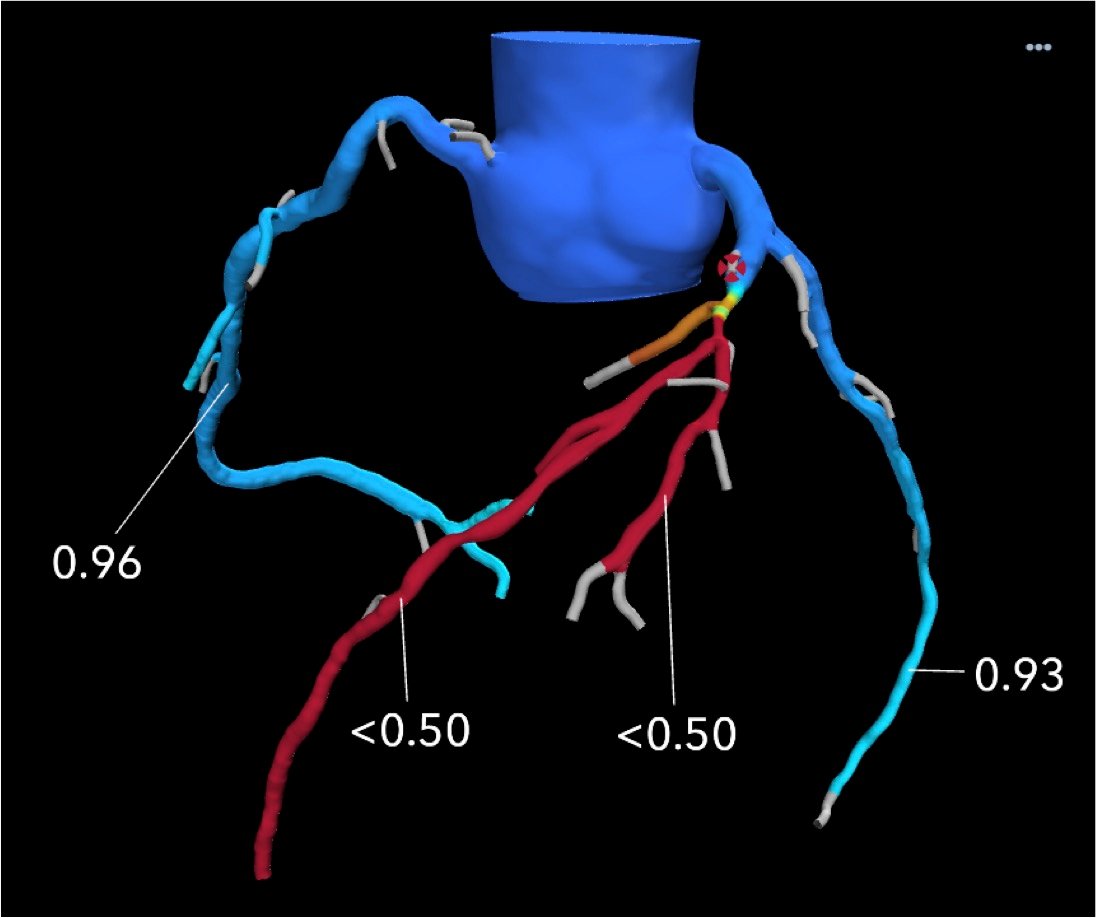 Tufts uses FFR-CT evaluations on non-emergency chest pain patients to reduce the need for diagnostic catheterizations. The center also uses the technology for intermediate-risk patients where it is not certain how physiologically severe a stenosis is.
Tufts uses FFR-CT evaluations on non-emergency chest pain patients to reduce the need for diagnostic catheterizations. The center also uses the technology for intermediate-risk patients where it is not certain how physiologically severe a stenosis is.
"There is a new type of emergency room patient with a gray zone sensitivity for troponins. It's not really negative where you can just send the person home, but it's clearly positive where you would admit them. It's uncertain. That's the kind of patient who I think would benefit very much from a FFR-CT if there is an intermediate stenosis," explained Udelson.
Watch the VIDEO: Use of FFR-CT to Non-invasively Evaluate Coronary Lesion Severity, interview with James Udelson, M.D.
Related Tufts Medical Center Cardiovascular Innovation Content:
VIDEO: Tufts Uses a Hemodynamic Support Algorithm to Determine What Devices to Use — Interview with Navin Kapur, M.D.
VIDEO: Remote Smartphone Access to Impella Consoles Aids Patient Care and Tech Support — Interview with Richard Botto, CVT, RCSA, chief cardiovascular technologist, and Melissa Smith, RT
VIDEO: Occluding the SVC as a Reset Button in Heart Failure — Interview with Navin Kapur, M.D.
VIDEO: Optimizing Platelet Inhibition in the Cath Lab at Tufts Medical Center — Interview with Carey Kimmelstiel, M.D.
VIDEO: The Role of Intensivists on the Cardiac Critical Care Team — Interview with Haval Chweich, M.D.
VIDEO: New Research in Cardiogenic Shock at Tufts Medical Center — Interview with Navin Kapur, M.D.
VIDEO: Overview of the Interventional Labs at Tufts Medical Center — Interview with Richard Botto, CVT, RCSA
VIDEO: Developing a Heart Failure Care Team — Interview with James Udelson, M.D.
VIDEO: Transcatheter PFO Closure to Prevent Stroke and Migraines — Interview with Carey Kimmelstiel, M.D.
VIDEO: Door-to-Unloading (DTU) Trial May Change STEMI Care — Interview with Navin Kapur, M.D.
VIDEO: Use of FFR-CT to Non-invasively Evaluate Coronary Lesion Severity — Interview with James Udelson, M.D.
VIDEO: Overview of the Structural Heart Program at Tufts Medical Center — Interview with Charles D. Resor, M.D.
VIDEO: Tufts Medical Center Spearheads Innovation With its Preclinical Cath Lab — Lara Reyelt, veterinary technician and preclinical surgeon
VIDEO: Overview of the TAVR Program at Tufts Medical Center — Andrew Weintraub, M.D.
VIDEO: Use of a Temporary Pacing Lead in TAVR — Interview with Andrew Weintraub, M.D.
3 New Approaches to Reduce Heart Failure Readmissions
VIDEO: Septal Ablation to Treat Hypertrophic Cardiomyopathy — Interview with Carey Kimmelstiel, M.D.
Additional videos and articles on Tufts Medical Center Channel


 November 14, 2025
November 14, 2025 









