June 4, 2015 - Using tools that help physicians decide whether to use expensive imaging studies can help reduce the ordering of unnecessary tests, but implementation in real-world settings has many challenges, according to a new RAND Corp. study.
The tools, computer-based programs that match a patient's characteristics against treatment criteria and recommend a treatment, helped increase the proportion of tests for Medicare fee-for-service patients rated as appropriate, according to findings published in the June 2 edition of the Journal of the American Medical Association.
However, the tools were not able to assign appropriateness ratings for a majority of tests because no appropriateness criteria were available for a particular test, or because the tool itself was not able to find matching criteria.
"The increase in orders rated as appropriate is promising, but the number of tests that were not rated indicates there is room for further improvement of these tools," said Peter S. Hussey, lead author of the JAMA article and a senior policy researcher at RAND, a nonprofit research organization. "In our full report to Congress, we've outlined a series of improvements to the decision support tools that we expect can lead to greater reductions in unnecessary tests."
The increasing use of advanced medical imaging such as magnetic resonance imaging (MRI) and computed tomography (CT) is often cited as a key driver of increasing medical costs. Beginning in 2017, federal rules will require that decision support tools must be used before ordering any advanced diagnostic imaging study paid for by Medicare.
The Medicare Imaging Demonstration was sponsored by the Centers for Medicare and Medicaid Services. The RAND Corp. conducted an independent evaluation of the effectiveness of the demonstration, testing whether exposing physicians to appropriateness guidelines for advanced imaging would reduce or eliminate unnecessary tests.
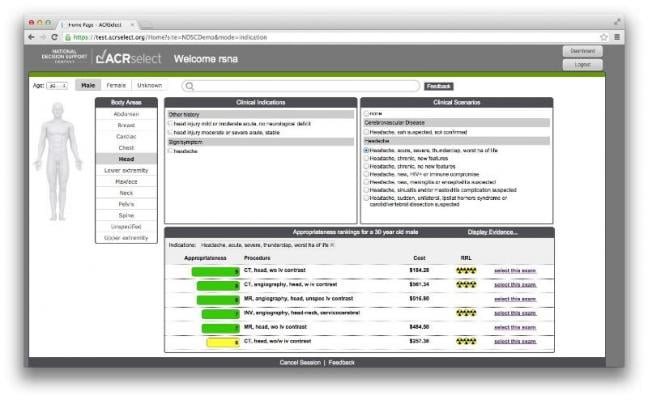
National medical specialty societies developed the appropriateness criteria using expert panels that reviewed evidence and completed a structured rating process. The same appropriateness criteria were loaded into the clinical decision support tools for all participating clinicians.
During the two-year study, 3,340 clinicians placed 117,348 orders for advanced diagnostic imaging procedures. Most orders were unable to be matched by the clinical decision support system to appropriateness criteria because no appropriateness criteria were available for a particular test, or because the tool itself was not able to find matching criteria.
However, there was a small increase in the percentage of orders rated appropriate between the baseline and intervention period, which indicates a successful intervention because fewer patients were exposed to clinically inappropriate, potentially harmful expensive tests.
"In response to these findings, we recommend that clinical decision support efforts should focus on tools that help clinicians perform their work more efficiently and effectively," said Katherine Kahn, M.D., principal investigator on the study, senior natural scientist at RAND and professor of medicine at the David Geffen School of Medicine at the University of California-Los Angeles (UCLA). "We need a more-comprehensive set of evidence-based guidelines that cover a greater proportion of advanced imaging orders for Medicare patients, and provide better methods for communicating feedback to clinicians."
Researchers note that the benefits of the design of this study are the scale and the diversity of settings. The participating organizations were located in eight states and included three academic medical centers, two integrated delivery systems, one group of independent practices in a single geographic area and one group of independent practices recruited by a radiology benefits management organization.
For more information: www.rand.org/health


 December 23, 2025
December 23, 2025