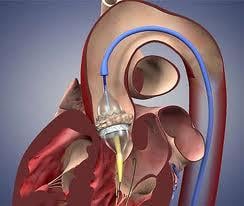
May 1, 2012 – The Centers for Medicare and Medicaid Services (CMS) announced today it will now cover transcatheter aortic valve replacement (TAVR) for Medicare patients under certain conditions. The coverage decision announced by CMS Acting Administrator Marilyn Tavenner offers important new technology to some of Medicare’s sickest patients.
Aortic valve replacements are used in patients whose aortic heart valves are damaged, causing the valve to narrow – a condition known as aortic stenosis. Once patients experience symptoms of aortic stenosis, treatment is critical to improve their chances of survival. Until recently, aortic stenosis has been treatable only through invasive surgery. In contrast, TAVR allows doctors to replace a patient’s aortic valve through a small percutaneous opening in the leg. This less invasive procedure gives patients who cannot undergo open-heart surgery a new way to repair their damaged heart valve.
“We are pleased with this decision and the increased access to treatment options it will provide,” said Tavenner. “This decision is particularly important as it highlights cooperative efforts among CMS, the U.S. Food and Drug Administration (FDA), the Agency for Healthcare Research and Quality, medical specialty societies and the medical device industry.”
This final national coverage decision is one of the first coverage decisions completed under a mutual memorandum of understanding between CMS and the FDA, a joint effort aimed at getting sometimes-lifesaving, new technology to patients sooner.
Because this technology is still relatively new, it is important that these procedures are performed by highly trained professionals in optimally equipped facilities. Therefore, this decision uses “coverage with evidence development,” which, as a condition of coverage, will require certain provider, facility and data collection criteria to be met. Such requirements are important to ensure beneficiaries receive the safest and most appropriate care.
The decision includes the Edwards Lifesciences' Sapien valve, which is the only FDA-cleared transcatheter aortic valve. Medtronic’s CoreValve is currently being reviewed in a U.S. pivotal trial that will be used to seek final FDA review.
Edwards commended CMS for issuing a flexible National Coverage Determination (NCD) that authorizes coverage for current and future indications approved by the FDA. Edwards also noted that, under the NCD, coverage would extend to qualified noninferiority or equivalence clinical studies. Additionally, important but small patient populations can be covered under a qualified study. The company continues to support the collection of TAVR outcomes data in a qualified national prospective registry.
"We endorse the NCD's emphasis on the use of multi-disciplinary heart teams in the treatment of TAVR patients," said Michael A. Mussallem, Edwards' chairman and CEO. "While we are still analyzing the policy and its lengthy supporting decision memorandum, we believe that this policy can provide appropriate and prompt access to treatment for these very sick patients."
The decision can be found at: www.cms.gov/medicare-coverage-database/details/nca-details.aspx?NCAId=257&ver=4&NcaName=Transcatheter+Aortic+Valve+Replacement+(TAVR)&bc=ACAAAAAAAAAA&


 December 24, 2025
December 24, 2025 









