July 26, 2012 — A new study shows promising data for a new method to image the source of atrial fibrillation (AF) and target its ablation to greatly reduce procedure time and substantially improve patient outcomes. The results of the CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) study were published online this week by the Journal of the American College of Cardiology.
The study found AF is typically caused by very few localized sources that cause disorganization in the remaining atria. Focal impulse and rotor modulation (FIRM) ablation to eliminate these sources was able to abruptly terminate or consistently slow persistent and paroxysmal AF in the vast majority of cases, and substantially improve long-term AF elimination over conventional ablation alone in this prospective case-cohort study. FIRM mapping may open the possibility for several patient-tailored therapies for AF in addition to ablation for this highly prevalent disease with major public health and societal impact. The authors concluded these results offer a novel mechanistic framework and treatment paradigm for AF.
Researchers said catheter ablation for AF is a promising therapy, but success is limited in part by uncertainty in the mechanisms that sustain AF. They developed a computational approach to map whether AF is sustained by several meandering waves or localized sources, then prospectively tested whether targeting patient-specific mechanisms revealed by mapping would improve AF ablation outcome.
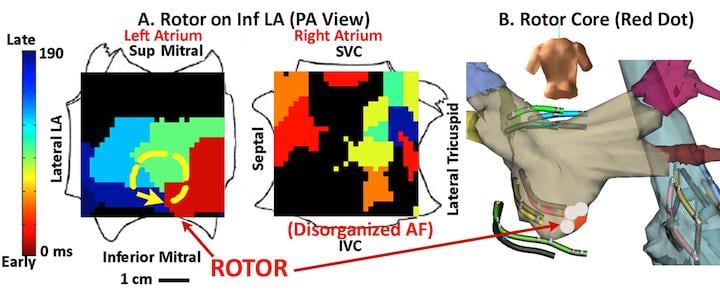
The study recruited 92 subjects during 107 consecutive ablation procedures for paroxysmal or persistent (72 percent) AF. Computational AF maps were generated intraprocedurally in the FIRM-guided group, and post-procedure in the FIRM-blinded group using a novel system (RhythmView, Topera Medical, Lexington, Mass). The FIRM maps of AF revealed electrical rotors defined as sequential clockwise or counterclockwise activation contours (isochrones) around a center of rotation emanating outward to control local AF activation, or focal impulses defined by centrifugal activation contours (isochrones) from an origin. Rotors and focal impulses showed limited spatial precession and were considered AF sources only if consistent in multiple recordings over 10 minutes (equating to thousands of cycles) to eliminate transient AF patterns of unclear functional significance.
In FIRM-guided subjects, ablation commenced with FIRM to eliminate sources. Radiofrequency energy was delivered using a 3.5 mm tip irrigated catheter (Thermocool, Biosense-Webster). The catheter was maneuvered to the basket electrode overlying each source, using fluoroscopy (or digital atrial mapping), and radiofrequency energy was applied for 15 seconds to 30 seconds. The catheter was moved within the area indicated by FIRM maps to represent the center of rotation or focal impulse origin until AF terminated or ablation time at that source reached less than or equal to 10 min, whichever came first (typically less than 5 minutes per source).
For more information: http://content.onlinejacc.org/article.aspx?articleid=1221482


 January 05, 2026
January 05, 2026 









