The Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) 2013 offers many new insights about the latest cardiovascular technologies and treatment techniques. These are my choices for the most innovative new or futuristic technologies highlighted on the show floor or in sessions at TCT 2013.
Transcatheter Mitral Valve Repair
Abbott Vascular’s MitraClip gained U.S. Food and Drug Administration (FDA) approval in October 2013, just prior to TCT. It is the first transcatheter mitral valve repair system cleared by the FDA. The system has long been highlighted as a futuristic technology at TCT, but this was the first time the device was shown with full U.S. market clearance. The device is delivered via a transseptal puncture to access the mitral valve. Under 3-D TEE guidance, it is deployed to clip the leaflets of the valve to aid in better diastolic sealing to prevent or reduce regurgitation.
Radiation Protection Cream
Interventionalists may frequently have their hands in the ionizing radiation field of angiography X-ray imaging system in the cath lab. Bloxr introduced its UltraBlox X-ray protection cream that physicians can coat their gloves in to reduce exposure by 30-50 percent without the use of lead. The cream was cleared by the FDA in mid-2013.
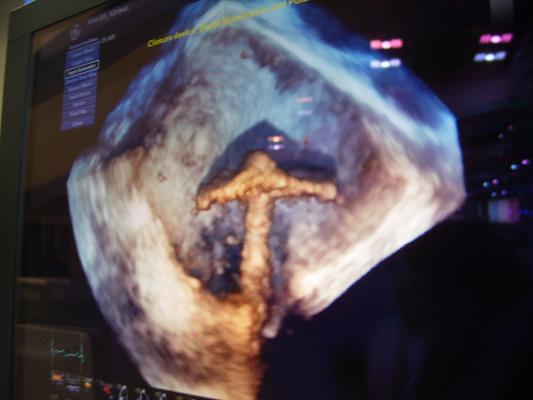
True 3-D Echo Imaging
GE Healthcare unveiled its XD Clear technology, which allows true 3-D visualization for echo images created by the Vivid E9 ultrasound system. It uses 3-D echo datasets to create a 3-D image on screen. When the operator wears a pair of PolarVision glasses, the image become true 3-D, making it much easier to differentiate depth. To further enhance this feature, GE also created Depth Illumination, which aids depth perception with the addition of synthetic shadowing. It makes anatomy look more like a real surgical view, as if the patient’s anatomy is exposed and seen under surgical lighting.
GE demonstrated these combined technologies from a recorded MitraClip procedure on 3-D/4-D TEE. It offered a very clear image of the device’s position, which is often not very clear on traditional 2-D or 3-D TEE.
3-D OCT Reconstruction
The FDA cleared St. Jude Medical’s Ilumien Optis PCI optimization system in October 2013, and it was shown for the first time in the United States at TCT. The system integrates both fractional flow reserve (FFR) and intravascular optical coherence tomography (OCT) imaging technology, and integrates several first-of-its-kind enhancements, including automated measurements and stent planning software tools. The OCT system can create real-time, 3-D reconstructions of the interior of a patient’s vessel, similar to a 3-D reconstruction created using computed tomography (CT) or magnetic resonance imaging (MRI). The reconstruction can easily visualize bifurcating vessels, stents struts and the interior luminal surface of the vessel. This makes visualization of the area being treated much easier for physicians.
Anatomical Intelligence to Speed Echo Workflows
Philips launched its Epiq Cardiac Ultrasound system, a new platform that utilizes artificial intelligence to help speed workflows for echocardiography. Echo is prone to a large amount of variability between operators. This system attempts to help standardize images and compensate for operator experience by showing ideal sample anatomical images of how standard diagnostic views should look so the operator can orientate a 3-D image dataset to match.
This is the initial rollout of Philips anatomical imaging, enabling about 25 percent of what the company eventually hopes will be a system that can identify optimal views automatically from 3-D echo datasets. The focus of anatomical intelligence component of this first release of Epic is to optimize mitral valve exams. Pictures show what a view of a mitral valve should look like and shows the points where measurements should be taken. The operator just needs to match up the images from their exam and match the measuring points. Philips says this can help cut the amount of time these exams take from 10 down to about two minutes.
Holograms in the Cath Lab
In recent years, angiography imaging systems vendors have partnered with other vendors to offer the latest in cath lab technology integration. Philips and its new partner Realview showed demonstrations of how real 3-D holograms might be incorporated in a cath lab to enhance procedural guidance and interventional planning. The Realview system allows users to take 3-D images created from CT datasets and slice and rotate these images in real time, in mid-air, without the need for special glasses.
Catheter Delivered Pacemaker
The world’s smallest pacemaker, also the first leadless and transcatheter delivered pacermaker, made its first debut at TCT. St. Jude Medical recently acquired Nanostim Inc., which received CE mark clearance in Europe for its device.
The Nanostim pacemaker is less than 10 percent the size of a conventional pacemaker, about the size of a large vitamin capsule. The small size of the device and lack of a surgical pocket, coupled with the exclusion of a lead, improves patient comfort and can reduce complications, including device pocket-related infection and lead failure. The elimination of the visible lump and scar at a conventional pacemaker’s implant site, in addition to the removal of patient activity restrictions that may prevent the dislodgement or damage to a conventional lead, will potentially improve the quality of life for patients with this technology by allowing most to continue living active, uninhibited lifestyles.
While the focus of TCT is interventional therapies, not electrophysiology, the device’s catheter delivery method makes it possible for interventional cardiologists to implant the system, especially in regions where there is a shortage of electrophysiologists — for example, in developing countries.
Next Generation Sapien 3 Valve
Edwards Lifesciences showed its Sapien 3 valve on the show floor and was mentioned in all sessions concerning new transcatheter aortic valve replacemebt (TAVR) technology. The valve design offers enhancements over the original Sapien and Sapien XT valves, including a lower profile, a fabric cuff intended to reduce paravalvular leak and new delivery systems.
Edwards received conditional investigational device exemption (IDE) approval from the FDA in August 2013 to initiate a clinical trial of the Sapien 3. The trial will study the valve in the treatment of high-risk and inoperable patients with severe symptomatic aortic stenosis. It will enroll up to 500 patients treated with one of three delivery techniques: transfemoral, transapical or the transaortic approach through a small incision in the chest and aorta. Edwards anticipates the trial will have a one-year composite endpoint compared to previous Sapien valves.
New Percutaneous VAD Technology
Abiomed showed two new work-in-progress percutaneous ventricular assist devices (VADs) at TCT. The first is the Impella Pediatric, which is a miniaturized version of the adult Impella 2.5 device. This new version can better fit pediatric anatomy, especially the smaller aortic arch.
The second device is the Symphony implantable counter pulsation device for heart failure patients. Similar to an intra-aortic balloon pump (IABP), the system augments the heart’s own pumping action for patients with Class III heart failure to help prevent them from progressing into Class IV.
Bioresorbable Electronics
Discussion in sessions shed light on the new technology of bioresorbable microelectronics. It is possible to print circuit boards made of magnesium on a polylactic acid bioresorbable polymer base to create temporary implantable devices that are engineered to dissolve after a specified period of time. Uses might include a hemodynamic blood pressure monitor attached to implantable devices, such as heart valves.
Electrolithoplasty to Crack Calcium
A future alternative to atherectomy or scoring balloons to tackle heavily calcified atherosclerotic lesions might be a balloon catheter that uses electromagnetic impulses to crack calcium. The technology is already used to breakup kidney stones. Todd J. Brinton, M.D, clinical associate professor of Medicine (cardiology), fellowship director program in biodesign, Stanford University, demonstrated proof of concept in videos shown during his presentation on the technology, where egg shells suspended in gelatin were cracked using a prototype catheter.
3-D Printing of Implantable Devices
Mayo Clinic is investigating the possibility of using commercially available 3-D printers to create customized implantable devices, such as heart valves or new blood vessels. The technology currently allows 3-D, CT or MRI image reconstructions of vessels and heart valves to be printed in plastic and rubber. Mayo clinic is looking at using biocompatible printing material to be able to print a new heart valve.
Theoretically, a patient’s 3-D CT scan data can be used as a basis to size and take a diseased valve and reconstruct it using software. It can then be printed and implanted in a patient, rather than using currently available one-size fits all devices.
The 3-D printer technology has been displayed at TCT for several years with the University of Colorado’s Hands on Hearts collection of 3-D printed plastic hearts, created from patient’s CT scans.
TCT Innovates Scientific Symposia
TCT itself was quite innovative this year, eliminating its massive printing of more than 10,000 show and session guides and instead issuing all attendees a Samsung Galaxy tablet computer. The tablet was preloaded with an app to navigate all the TCT sessions and events, and offered features such as the ability to answer polls during sessions, ask the presenters questions during sessions, download presentation slides and message colleagues. While this offered a level of interaction never before possible with traditional paper guides, it required an army of people detailed to IT helpdesks to walk users through how to set up the devices and navigate the app.