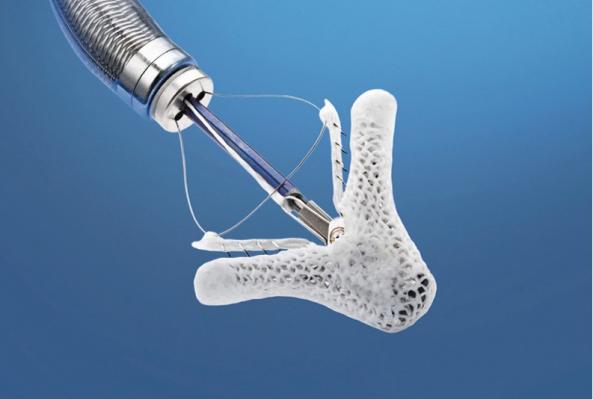
FDA Advisory Committee Supports MitraClip for Patients With Significant Mitral Regurgitation Who Are Too High Risk for Surgery
March 22, 2013 — Abbott announced that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee has voted by majority (Yes: 5, No: 3) that the benefits of treatment with the MitraClip device outweigh its risks in patients with significant symptomatic mitral regurgitation (MR) who have been determined by a cardiac surgeon to be too high risk for open mitral valve surgery and in whom existing co-morbidities would not preclude the expected benefit from correction of the MR.
Abbott's MitraClip device, which received CE mark in 2008 and is commercially available in Europe and other international markets, is an investigational device in the United States. The device is delivered to the heart through the femoral vein, a blood vessel in the leg, and is designed to reduce MR by clipping together a portion of the leaflets of the mitral valve to allow the heart to more efficiently pump blood.
"We appreciate the FDA's dedication of time and resources to convene this advisory panel for MitraClip and for its review of this new and novel technology for high surgical risk patients suffering from the debilitating symptoms of significant mitral regurgitation," said Charles A. Simonton, M.D., FACC, FSCAI, divisional vice president, Medical Affairs, and chief medical officer, Abbott Vascular. "
On the separate question of whether there is reasonable assurance the device is safe, the panel voted Yes: 8, No: 0. On the question of whether there is reasonable assurance of efficacy, the panel voted Yes: 4, No: 5. The FDA will take into account the panel's advice in making its decision on whether to approve the MitraClip for the treatment of significant MR in the United States. The company expects a decision later this year.
The committee's recommendation followed a review of data from a large and growing body of clinical evidence (EVEREST II, EVEREST II High Risk and REALISM) in which the MitraClip therapy demonstrated positive and consistent results for high surgical risk patients suffering from the debilitating symptoms of significant MR, including a safe procedure, reduction in MR, reverse left ventricular remodeling, improvement in heart failure symptoms, improvements in quality of lifeand reduced rates of re-hospitalization.
For more information: www.abbott.com
Reference:
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. “Burden of valvular heart diseases: a population-based study.” Lancet. 2006 Sept. 16; 368(9540):1005-11.


 December 24, 2025
December 24, 2025 









