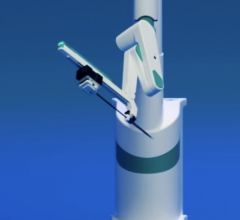
July 25, 2012 — Corindus Vascular Robotics today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the CorPath 200 robotic-assist system for use in percutaneous coronary interventions (PCI).
“The FDA clearance of the CorPath system will truly change the way I am able to practice,” said Joseph P. Carrozza Jr., M.D., chief of cardiovascular medicine at St. Elizabeth’s Medical Center in Boston. “As interventional cardiologists, we perform our procedures using X-ray guidance and are cognizant that throughout our careers we will be exposed to a high amount of radiation. In the past, we have relied on heavy lead aprons to protect us from radiation, but the physical stress of wearing these aprons can lead to back pain, fatigue and orthopedic injuries.”
Recent data published in Catheterization and Cardiovascular Intervention journal indicated that an interventional cardiologist’s daily exposure to radiation and the physical stresses inherent in the cath lab can lead to occupational health risks — including orthopedic problems, cataracts and cancer. CorPath PRECISE Trial was a prospective, single-arm, multi-center study, which served as the basis for the submission of an FDA clearance application. It demonstrated that robotically assisted PCI is safe and feasible for patients. PCI was successfully completed without having to convert to manual PCI in 98.8 percent of patients and without device-related complications. The overall procedure success rate was 97.6 percent. Additionally, the trial found that robotic-assisted PCI can make the procedure safer for the interventional cardiologist by reducing the radiation exposure by 95 percent when performing the procedure with the CorPath 200 system.
“I was impressed with its performance and the precise control of the interventional devices, including manipulating the guidewire and stent, being able to move the devices precisely in increments as small as one millimeter,” said Giora Weisz, M.D., director of clinical research at the Center for Interventional Vascular Therapy at NewYork-Presbyterian Hospital/Columbia University Medical Center, New York. “Working with this robotic technology is very intuitive and the PRECISE trial demonstrated its applicability in today’s cath lab environment. I strongly believe robotic-assisted PCI will enhance the way we are conducting PCI, and we are looking forward to adopting it in our everyday practice.”
The CorPath 200 is the first and only robotic-assisted procedure to allow for controlled placement of coronary guidewires and stent/balloon catheters from an optimized interventional cockpit. The lead-lined cockpit protects the interventional cardiologist from harmful radiation exposure, and the seated position in front of monitors may provide enhanced view of the angiography screen while reducing fatigue and minimizing head, neck and back strain.
In June, Hansen Medical Inc. received FDA 510(k) market clearance for its Magellan Robotic System, the first robotic navigation system to more precisely guide peripheral artery disease (PAD) interventions in the cath lab.
For more information: www.corindus.com


 January 27, 2026
January 27, 2026