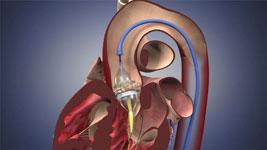
The Edwards Sapien transcatheter aortic valve is pending FDA clearnance. The valve can be delivered both transfemorally (pictured here) or transapically in a hybrid OR.
In a closely watched move – viewed as a big step toward a paradigm shift in how heart valves are repaired in the United States – a U.S. Food and Drug Administration (FDA) advisory panel in July recommended approval of the Edwards Lifesciences Sapien transcatheter heart valve.
The FDA Circulatory System Devices Panel recommended the valve specifically for the treatment of patients too sick to undergo the standard therapy of surgical valve replacement. While the FDA is not required to follow the approval recommendation from the committee, the agency takes the advice into consideration when rendering its final decisions. The FDA will likely consider final market clearance of the Sapien later this year.
The valve is delivered using minimally invasive, percutaneous cath lab delivery techniques. It is designed for treatment of patients with severe symptomatic native aortic valve stenosis who are unable to undergo valve replacement surgery. However, it is widely anticipated that transcatheter aortic valve implantation (TAVI) in the future will replace the current standard of open-heart surgery to repair or replace heart valves.
“The FDA panel’s recommendation further reinforces the value of TAVI for inoperable patients,” said Christopher J. White, M.D., FSCAI, SCAI president, chairman of the department of cardiology and director of the Ochsner Heart and Vascular Institute in New Orleans. “For more than 30 years, advances in interventional cardiology have helped improve survival for patients with heart disease. TAVI is an important advancement…”
The Sapien valve is an investigational device in the United States and not yet available commercially. It received CE mark approval for European commercial sale in late 2007. Edwards submitted an FDA pre-market approval (PMA) application in the fall of 2010, based on data from the inoperable cohort of the PARTNER trial. This cohort compared the outcomes of 358 patients after treatment with either standard medical therapy (usually balloon aortic valvuloplasty) or the Sapien valve delivered transfemorally. The results, released at Transcatheter Cardiovascular Therapeutics (TCT) 2010 last fall, were so overwhelmingly positive for the valve that top cardiologists questioned the ethics of continuing to randomize patients to ineffective valvuloplasty or medical therapy treatments.
“We are pleased with the panel’s strong recommendation for approval, and would like to thank them for their comprehensive and thoughtful review of the data presented from the PARTNER trial,” said Michael A. Mussallem, Edwards’ chairman and CEO. “This represents another important step on the path to what we hope will lead to FDA approval of Sapien.”
The Success of the PARTNER Trial
Speaking in support of the device at the panel meeting were American College of Cardiology (ACC) President David R. Holmes Jr., M.D., FACC; Michael J. Mack, M.D., FACC, president of The Society of Thoracic Surgeons (STS), and Augusto Pichard, M.D., FACC, FSCAI, speaking for the Society for Cardiovascular Angiography and Interventions (SCAI). All of them urged the FDA to approve this recommendation based on the results of the PARTNER cohort B trial. (1)
The study found lower rates of mortality among patients who received TAVI (30.7 percent vs. 50.7 percent, p<0.001) and lower rates of a composite endpoint of death from any cause or repeat hospitalization (42.5 percent vs. 71.6 percent, p<0.001). TAVI was associated with a significant reduction in symptoms.
However, TAVI was also associated with more neurological events, including strokes, major vascular complications and major bleeding events as compared to the standard therapy group. Part of this was attributed to the operator learning curve in mastering the techniques required for the new therapy. The trial was also performed without the use of aortic embolic protection devices, which are now being developed in hopes of reducing neurological and stroke events.
SCAI issued a statement saying the risk of stroke and vascular complications is always of concern, but the risk is offset by a dramatic benefit in survival. “For patients unable to receive surgery, the survival benefit of TAVI far outweighs the risk of complications and far exceeds the benefit of medical therapy,” the statement read.
“Patients with severe aortic stenosis have very few options and they need treatments like TAVI or they could die from their disease,” said Pichard, who is director of the cardiac catheterization lab at Washington Hospital Center and professor of medicine at Georgetown University. “The PARTNER B trial demonstrates TAVI significantly improves symptoms compared to standard treatments for patients who cannot receive surgery. We urge the FDA to approve this device and allow patients a treatment option that improves quality of life and extends lives.”
Preparing for a New Era
If TAVI is approved by the FDA and becomes available for use in the United States, SCAI believes it will be essential for cardiovascular societies to work together to define program accreditation criteria and develop rigorous training program standards to train physicians. SCAI also said there is a need to establish a nationwide registry to monitor patient complications from these procedures and develop standards for multidisciplinary team approaches to providing TAVI.
“To ensure rational and responsible dissemination of this new technology, government, industry and medicine will need to work in harmony,” Holmes said in written comments submitted to the FDA before Wednesday’s hearing. “We look forward to this opportunity to work together to make high-quality patient care available to the appropriate patient population in the appropriate setting from appropriately experienced operators conducting the necessary followup.”
In his written comments, Holmes urged the panel to consider post-market research and surveillance, such as through a national database like the National Cardiovascular Data Registry (NCDR).
Currently, SCAI is leading the development of a multi-society policy statement on institutional and operator requirements to define the essential criteria for optimal patient outcomes with the ACC, STS and the American Association for Thoracic Surgery (AATS).
Reference:
1. Leon, M.B., et al., “Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery.” New England Journal of Medicine. Oct. 21, 2010; 363(17):1597-607



 December 24, 2025
December 24, 2025 









