October 12, 2012 — Boston Scientific announced it has enrolled the first patient in the REPRISE II clinical trial to evaluate the safety and performance of the Lotus valve system in up to 120 patients with severe aortic valve disease. This international multicenter study includes 15 sites in Australia, France, Germany and the United Kingdom. The Lotus valve system is the first transcatheter aortic valve replacement (TAVR) device of its kind that is designed to minimize aortic regurgitation (leaking). The device is both fully repositionable and retrievable prior to release, offering predictable and precise placement. The results of the REPRISE II trial are expected to be used to support CE mark and other international regulatory approvals.
"We were encouraged by the promising results of the REPRISE I clinical trial completed earlier this year, and we are therefore very confident about evaluating the safety and performance of the Lotus Valve in a larger patient cohort," said Ian Meredith, professor and director of Monash Heart at Monash Medical Center in Melbourne, Australia, and the principal investigator of the REPRISE II trial. "The Lotus Valve has a number of important features which address some of the limitations observed with the first generation devices. The ease of use, predictable and precise positioning, and ability to fully reposition and retrieve the Lotus Valve offer the operator considerable reassurance and control. These features, along with the minimized risk of paravalvular leakage, may lead to improved clinical outcomes."
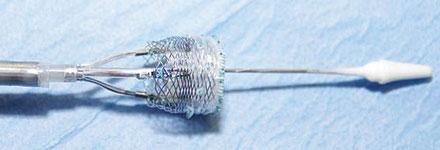
The Lotus valve system is a differentiated second-generation TAVR technology, which consists of a pre-loaded, stent-mounted tissue valve prosthesis and catheter delivery system used for guidance and percutaneous placement of the valve. The low-profile delivery system and introducer sheath are designed to enable predictable and precise placement, as well as bidirectional atraumatic repositioning and retrieval at any time prior to release of the aortic valve implant. The device also employs a Adaptive Seal feature designed to minimize the incidence of paravalvular regurgitation, which has proven to be a predictor of mortality.
Current study enrollment requires that patients have severe calcific aortic stenosis, are aged 70 years or older, and are considered to be at high surgical risk, defined as an STS (Society of Thoracic Surgeons) mortality score greater than 8 or by the consensus opinion of the heart team. The REPRISE II study is designed to study two valve sizes, 23 mm and 27 mm, and have a five-year follow-up. Beyond that, it will assess for other endpoints recommended by the Valve Academic Research Consortium (VARC) and regulatory agencies. Study enrollment is expected to be completed in the first half of 2013.
"There is a high need for continued evolution of device technology to treat people with severe aortic valve disease," said Keith D. Dawkins, M.D., global chief medical officer for Boston Scientific. "We are looking forward to advancing the Boston Scientific valve program with REPRISE II, as we believe the Lotus valve system is a unique technology that will offer interventional cardiologists greater precision and control in deployment which, in effect, may simplify the implantation procedure and lead to improved patient outcomes."
The Lotus Valve System is an investigational device, limited by applicable law to investigational use and not available for sale. The device was developed by Sadra Medical, which Boston Scientific acquired in 2011.
For more information: www.sadramedical.com


 December 24, 2025
December 24, 2025 









