June 30, 2015 - Cardiac Dimensions announced the first patients have been enrolled in the REDUCE FMR clinical trial. REDUCE FMR is a prospective, double-blind, randomized multi-center trial, evaluating the company's minimally-invasive Carillon Mitral Contour System.
Involving up to 20 leading hospitals in Europe and Australia, REDUCE FMR will randomize 120 patients and is designed to establish Carillon as the gold standard treatment for functional mitral regurgitation (FMR) - a condition in which blood flow to the body is reduced due to an abnormally enlarged mitral valve. All patients enrolled in the study are on an optimized heart failure medication regimen and are then randomized into two groups: one additionally treated with the Carillon device and the second remaining on an optimized regimen of heart failure medications, the present gold standard.
The study design contains some unique elements aimed at optimizing recruitment and enrollment, including a 3:1 randomization ratio allowing for more data to be collected with the Carillon device and a crossover registry which allows control patients to receive Carillon treatment at the end of their 12-month follow up. A built-in exercise echocardiographic sub-study will further evaluate the device's ability to reduce mitral regurgitation, improve functional capacity and quality of life as well as induce reverse ventricular remodeling in a symptomatic heart failure patient population both at rest and during exercise.
The REDUCE FMR clinical trial follows three successful multi-center studies, featuring the Carillon device – the AMADEUS, TITAN and TITAN II trials. "My experiences with Carillon have been extremely positive. As an investigator in the last two multi-center trials involving the Carillon therapy I've seen significant clinical improvement in patients who receive the device," said Janusz Lipiecki, M.D., of Clinique Pí´le Santé République in Clermont Ferrand, France. "In this latest landmark trial, we expect to firmly establish the magnitude of benefit that patients receive from Carillon."
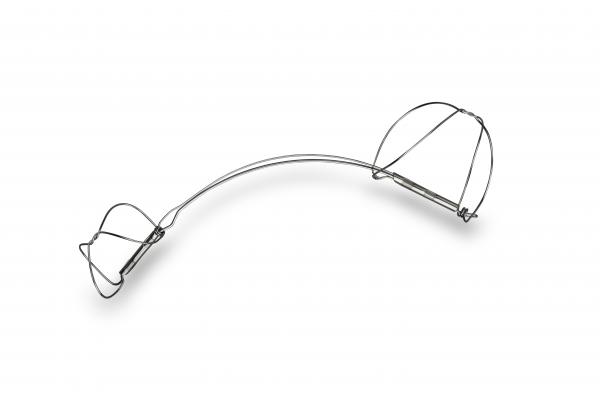
The Carillon Mitral Contour System is a percutaneous mitral annuloplasty treatment option that can be deployed rapidly and safely utilizing standard interventional techniques. The implantable device consists of a distal anchor and a proximal anchor connected by a shaping ribbon and utilizes the heart's venous anatomy to reshape the mitral annulus. This approach allows for reduction of the dilated annulus, addressing a root cause of FMR.
For more information: www.cardiacdimensions.com


 December 24, 2025
December 24, 2025 









