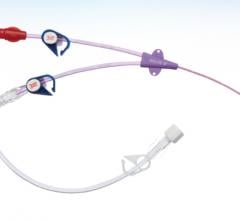
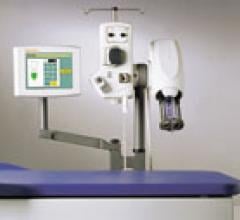
Covidien's Optivantage injector system is radiofrequency identification (RFID) enabled to automatically record information from the syringe and sends it to the injector head.
Automated contrast media injectors control contrast dosage, record the amount used and speed injections to keep up with today’s fast computed tomography (CT) scanners. They also warn of potential hazards, such as air embolisms or extravasations.
Key Differences in Injectors
Angiography systems typically use single-head injectors with one syringe, while CT systems use a dual-head injector with two syringes. The double syringes allow a large, fast initial bolus of contrast and a second syringe filled with a follow-up saline-contrast flush to keep the contrast flowing in the body during CT exams.
The contrast delivery is more controlled and efficient when using a dual-head power injector and is extremely quick, enabling the high speeds possible with newer multidetector CT scanners.
CT injectors use venous access, so air embolisms are not as big an issue, as small bubbles are expelled through the lungs. Angiography systems inject contrast into arteries, where air embolisms are a serious risk. For that reason, many cath lab injectors have air embolism detectors.
CT imaging studies usually require high-flow, high-volume, fixed-rate injections delivered with relatively high pressures. However, in the interventional suite, procedures need low, variable flow rate injections. Injectors used with magnetic resonance imaging (MRI) are engineered to prevent electrical interference in the magnetic field. They use a hydraulic control system or include shielding of the electrical controller.
In the Cath Lab
Automated injectors are replacing traditional hand injection syringes and stopcocks. Systems may allow variable flow, so adjustments can be made depending on whether contrast is being delivered to small coronary vessels or to larger peripheral vessels. These systems also track the amount of contrast used in each patient during a procedure, helping to prevent contrast-induced nephropathy (CIN) in high-risk patients.
Acist offers a hand controller for precise administration of contrast to allow clinicians to step back from the radiation source during imaging to reduce radiation exposure.
In August, Acist reached a milestone with its contrast injection system technology for cardiovascular angiography when it announced its system was used to help diagnose and treat 10 million patients.
“The Acist contrast delivery system was developed to help doctors perform angiography more efficiently and, in turn, facilitate timely and appropriate treatment,” said Robert Wilson, M.D., inventor of the Acist system and professor of interventional cardiology at the University of Minnesota. “The system shortens procedures, decreases the amount of dye injected into patients and reduces radiation exposure for healthcare professionals and patients.”
Medrad received 510(k) approval from the U.S. Food and Drug Administration (FDA) for its new Mark 7 Arterion angiography injection system after the deadline for this chart.
Workflow Improvements
Automated injectors allow the injection rate to be programmed and provide other features to aid clinicians. Some offer extravasation sensors to warn if the injector needle is embedded in tissue instead of inside the vessel. Injector control of contrast delivery timing can also be leveraged to optimize imaging. If timed properly, a system can help limit the number of scans necessary by eliminating repeats because of problems with insufficient contrast delivery.
Data management systems offered for some injectors provide data to measure workflow efficiencies, imaging optimization and patient safety. The systems can also integrate preprogrammed multidetector CT protocols. Other features may include auto initialize, auto fill and auto purge, as well as air embolism protection.
Comparison Chart
This story was an introduction to a comparison chart for contrast media injector systems marketed cardiac imaging or angiography systems. To find the chart, click on the "comparison charts" tab at the top of the page. Participants in the chart include:
- Acist Medical Systems, www.acist.com
- Covidien, www.covidien.com
- Medrad Inc., www.medrad.com
- Nemoto Kyorindo, www.nemoto-do.co.jp/nemoto_en


 January 11, 2024
January 11, 2024