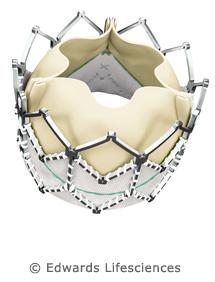
Lower-Profile Sapien XT Transcatheter Heart Valve Associated With Improved Procedural Outcomes
March 18, 2013 — Edwards Lifesciences Corporation announced that preliminary results from The PARTNER II clinical trial demonstrated similar one-year outcomes in mortality and major clinical events between the Edwards Sapien XT transcatheter aortic valve and the Edwards SAPIEN valve, yet fewer vascular events with the lower-profile Sapien XT valve. These data from The PARTNER II Trial studying transcatheter aortic valve replacement (TAVR) in inoperable patients with severe, symptomatic aortic stenosis were presented as a late-breaking clinical trial at the American College of Cardiology's (ACC) 62nd Annual Scientific Session in San Francisco.
The PARTNER II Trial enrolled 560 patients deemed inoperable for traditional open-heart surgery at 28 hospitals in the United States between April 2011 and February 2012. Patients were randomized to receive one of the two Edwards transcatheter aortic heart valves: 276 received the Sapien valve, and 284 received the Sapien XT valve.
The U.S. Food and Drug Administration (FDA) approved the Sapien valve in November 2011 for the treatment of inoperable patients, and expanded the indication to high-risk surgical patients in October 2012. The Sapien XT valve is an investigational device not yet available commercially in the United States.
Edwards anticipates submitting data from the inoperable cohort (Cohort B) of The PARTNER II Trial to the FDA in the second quarter. The company expects to complete enrollment in the intermediate risk cohort (Cohort A) of The PARTNER II Trial mid-year.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









