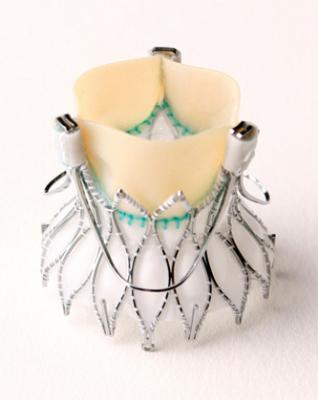
February 28, 2013 — Medtronic Inc. announced European CE (Conformité Européenne) mark approval for its Engager Transcatheter Aortic Valve Implantation (TAVI) System with transapical delivery catheter. The system is indicated to treat patients with severe aortic stenosis who are at high or extreme risk for surgical aortic valve replacement (SAVR).
The new valve demonstrated positive clinical outcomes in its European Pivotal Trial. Results from the multi-center trial, which were presented during late-breaking trial sessions at the recent European Association for Cardio-Thoracic Surgery and the Society of Thoracic Surgeons annual meetings, revealed high rates of procedural success, minimal paravalvular leak (PVL) and continuing clinical benefits for patients over time.
In the trial, the Engager valve was delivered transapically and had 94.3 percent overall device success (according to Valve Academic Research Consortium modified definitions). There were no procedures requiring a second valve and no occurrences of valve embolization, coronary obstruction or device malposition. No patients had moderate or severe PVL at six months, as measured by an independent echocardiography core lab. In addition, while most patients (88 percent) were NYHA Class III or IV at baseline, at six months 82 percent of patients had improved to NYHA Class I or II.
“The Engager valve has demonstrated exceptional clinical results, and by adding it to our transcatheter valve portfolio, we are providing heart teams with more options for achieving the best outcomes for every patient with severe aortic stenosis,” said John Liddicoat, M.D., senior vice president of Medtronic and president of the Medtronic structural heart business.
The Medtronic Engager System is not available in the United States.
The Engager valve uses a minimally?invasive delivery system via a catheter inserted in the apex (the lower, pointed end) of the heart. The valve is comprised of bovine tissue leaflets and a self?expanding nitinol frame designed to promote annular sealing to minimize paravalvular leak. Control arms simplify implantation, and the supra-annular valve positioning facilitates leaflet coaptation (connections) in non-circular anatomy for optimal hemodynamic performance. A direct aortic delivery system for Engager will be introduced in the future.
For more information: www.medtronic.com


 December 24, 2025
December 24, 2025 









