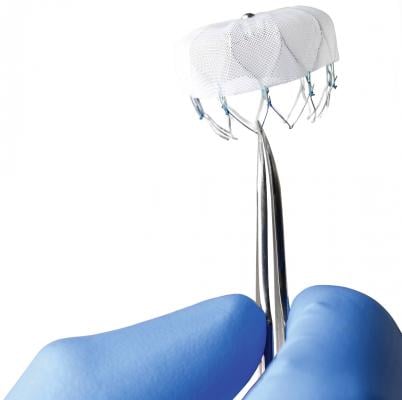
The Boston Scientific Watchman device is currently the only transcatheter LAA occluder cleared for use in the United States.
Atrial fibrillation (AF) affects nearly 6 million Americans and the condition puts them at significantly greater risk of stroke. These strokes resulting from the embolization of left atrial appendage (LAA) thrombi account for up to 25 percent of the 700,000 cerebrovascular strokes annually in the United States.[1] Many experts believe the LAA is the culprit in a much higher percentage of strokes. Stasis, hypercoagulability and endothelial dysfunction are said to contribute to LAA thrombus formation in AF patients.[1]
Oral anticoagulants, mainly warfarin, have been the standard of care for reducing the risk for stroke in these patients, but many patients cannot tolerate these medications because of the incidence of bleeding and need for bi-weekly blood tests. Patient adherence to taking these drugs has also led to gaps that can lead to stroke. This has led to the use of LAA occlusion (LAAO) by open surgical or transcatheter methods as a non-pharmacologic alternative to warfarin.
Transcatheter LAA Occlusion
The Watchman device, made by Boston Scientific, is the first and only transcatheter LAAO device approved by the U.S. Food and Drug Administration (FDA). It was cleared in the United States in March 2015, but had previously gained European CE mark approval in 2005. It is inserted through the femoral vein in the leg and fed into the right atrium of the heart. A transseptal puncture is used to enter the left atrium and allow a clear line of site to access and deploy the device in the LAA.
To gather and monitor the clinical data on the Watchman and subsequent transcatheter LAAO devices, the American College of Cardiology (ACC) announced the creation of the Left Atrial Appendage Occlusion Registry in December and it began to capture data in early 2016. This data will help assess real-world procedural indications, outcomes and short- and long-term safety of LAAO procedures and devices. The registry also serves as the formal FDA-mandated post-approval study for the Boston Scientific Watchman device.
However, this ACC registry did not capture the first 15 months of data from the first 1,683 patients implanted since Watchman’s FDA clearance. However, the FDA required a trained Watchman specialist to be on-hand to help with implantations, and they kept detailed records of all these procedures. This early post-approval data was presented at the ACC.16 meeting by David Holmes, M.D., professor of medicine, Mayo Clinic College of Medicine and consultant, Department of Internal Medicine, Division of Cardiovascular Diseases.
“In this group, 70 percent of these patients were treated by physicians who had not been primary implanters before and 50 percent of the institutions had not been implant institutions before [in the PROTECT AF Watchman Trial],” Holmes explained. “That is important because it helps us identify the learning curve in newly trained physicians… The procedural success was 95 percent, and that is better than the original PROTECT AF Trial published in 2009.”
The data show the Watchman devices were recaptured and repositioned to properly seat it in about 25 percent of procedures. The study also found the average procedural time is about 50 minutes, which is the same as reported in PROTECT AF, Holmes said.
The Watchman implant procedure requires a transseptal puncture to provide a direct delivery line into the LAA. It was speculated early on that this may position electrophysiologists (EPs) to be more qualified to perform the procedure, since they conduct transseptal punctures on a regular basis. However, Holmes said the implantations since FDA approval have been performed about 50/50 between interventional cardiologists and EPs. He added that FDA and the Centers for Medicare and Medicaid Services (CMS) have encouraged these procedures be performed collaboratively between both specialties.
The new ACC (LAAO) registry joins eight other programs that comprise the ACC National Cardiovascular Data Registry (NCDR). “It is exciting that we are launching this registry at the beginning of implementation of this transformative therapy,” said Paul Varosy, M.D., FACC, FAHA, FHRS, director of cardiac EP at VA Eastern Colorado Health Care System, who is the lead physician for the dataset development work group for the LAAO Registry. “Clinicians, hospitals, payers and especially patients will benefit from a clear understanding of how these devices are being used in real-world practice. A registry like this can provide important tools in ensuring that the quality of care is as high as it can be for patients receiving these devices.”
Watchman LAA Occluder Technical Considerations
Several speakers offered advice on LAA occlusion using the Watchman at an ACC.16 session. Jason Rogers, M.D., clinical professor of cardiovascular medicine and director, interventional cardiology, UC Davis Medical Center, Sacramento, Calif., offered several suggestions.
He said the LAA will change in shape by as much as 2 mm due to dehydration, so he urges the use of a saline bolus to ensure proper sizing for the Watchman device. He said it takes about 20-30 Watchman implants before an operator becomes proficient at the procedure. He also suggests having a pericardiocentesis tray ready to use in case of procedural complications. Finally, he said the heart team working together on the patient need to be on the same page.
“LAA closure is a team effort, so you all need to use the same language,” Rogers said. This includes use of standard taxonomy that everyone understands for position and anatomy.
The shape of the LAA is also very important to consider, said Shephal K. Doshi, M.D., FACC, director of cardiac EP and pacing, Providence Saint John’s Health Center, Santa Monica, Calif. He explained the shape and size of the LAA, especially the ostium, can determine if a Watchman device can be easily placed or anchored properly.
The LAA anatomy comes in a wide variety of shapes and sizes. Some of the common types (which describe how they appear on angiography or computed tomography [CT] imaging) include the chicken wing, broccoli and windsock, each presenting anatomical challenges to LAA closure, and some do not work well with the Watchman. Doshi said the windsock shapes are the best suited for the Watchman. He also warned that prominent LAA trabeculations can cause deployment issues for the Watchman.
Determining the shape, orifice size, depth and width of the LAA is very important to define with CT imaging prior to a procedure and for procedural planning, said Jacqueline Saw, M.D., UBC, FRCPC, FACC, FAHA, FSCAI, program director, interventional cardiology fellowship program, Vancouver General Hospital, Canada.
She said some anatomy is just not suitable to anchor a Watchman device. Cardiac CT protocols for LAA imaging include the use of contrast and delayed enhanced imaging to help visualize the presence of thrombus, which will be seen as a filling defect. Dual-energy CT can also be helpful for LAA evaluations, since it makes thrombi easier to visualize.
“Envision how the device will fit into the LAA,” Saw said. “I think 3-D volume rendering imaging is very helpful for evaluating these structures.”
Medicare Coverage for LAA Occlusion
CMS announced in February it would begin covering percutaneous LAA closure therapy under specific criteria, as outlined in the agency's final National Coverage Determination (NCD). The decision provides consistent access to the Watchman LAA closure device as a non-pharmacological treatment option for stroke risk reduction for appropriate Medicare beneficiaries. CMS made the coverage change based on feedback from physicians and professional medical societies.
Prior to the CMS final decision, a number of private payers, including several Blue Cross Blue Shield plans, have updated their policies to now cover the Watchman device, Boston Scientific said.
LAAO Cost-effectiveness
A study published in the Journal of American College of Cardiology (JACC) determined the Watchman device is more cost-effective than warfarin and non-warfarin oral anticoagulants (NOACs) for stroke reductions in non-valvular atrial fibrillation patients.[1] The study, led by Vivek Reddy, M.D., director of cardiac arrhythmia services for The Mount Sinai Hospital and Mount Sinai Health System, New York, showed the Watchman achieved cost effectiveness relative to warfarin at seven years ($42,994/QALY), and NOACs achieved cost effectiveness relative to warfarin at 16 years ($48,446/QALY) when assessing Medicare beneficiaries over a 20-year period. The device became dominant (more effective and less costly) to warfarin at 10 years and dominant to NOACs at five years, with cost savings generated annually thereafter.
One of the main issues with the effectiveness of anticoagulation therapy is patient compliance.
“By its very nature, the Watchman device is not subject to patient adherence issues, since once implanted, the device provides lifelong stroke prophylaxis without the risk of complications associated with blood thinners,” Reddy explained. “This is crucial both in improving patient outcomes by reducing disabling strokes, as well as reducing healthcare costs.”
Researchers used a Markov model constructed from the perspective of CMS with a lifetime horizon defined as 20 years and three-month cycle length to evaluate the cost effectiveness of the three treatment strategies. Cost effectiveness was assessed annually to determine if there was an observable time horizon over which treatment options reached accepted levels of cost effectiveness.
LAA Technology News
Boston Scientific announced the first implants of its next-generation Watchman FLX device in Europe following CE mark approval last November. The FLX uses a closed-end design and has the ability to be fully recaptured and repositioned.
In July 2015, the FDA issued a safety warning about reports of patient deaths and other serious adverse events associated with the use of the SentreHeart Lariat Suture Delivery Device. It delivers a pre-tied suture loop through traditional open surgical procedures, or through an access port as small as 5 mm to close the LAA. The FDA said it identified 45 adverse events through June 30, 2015, that occurred in patients undergoing LAA closure procedures with the Lariat device and/or its associated devices. These reports describe six patient deaths and other serious medical complications, including laceration and/or perforation of the heart, complete LAA detachment from the heart, hemorrhage, low blood pressure, fluid collection around the heart (pericardial effusion), fluid collection around the heart that causes low blood pressure and decreased heart function leading to shock (cardiac tamponade), and fluid collection around the lung (pleural effusion). Of the 45 adverse events reported to the FDA, 34 (approximately 75 percent) resulted in the need to perform emergency heart surgery.
In April, the FDA cleared the AtriCure Inc. AtriClip PRO2 LAA Exclusion System. The new system has increased functionality that enhances the capability to occlude the LAA during minimally invasive surgical procedures. It features an ambidextrous locking and trigger-style clip closing mechanism, handle-based active articulation levers and a hoopless end effector. The ambidextrous locking and trigger-style clip closing mechanism allows the operator to maintain focus on the LAA while maneuvering the device. The handle-based active articulation levers allow the operator to steer the end effector without removing the device. The hoopless end effector enhances anatomical visualization and simplifies removal of the applier after deployment of the clip.
“The AtriClip PRO2 system provides easier placement of the proven AtriClip LAA occlusion technology,” said J. Michael Smith, M.D., of TriHealth Heart Institute in Cincinnati. “The new deployment system facilitates less invasive treatment of the LAA, including right chest approaches in conjunction with valve replacement and cardiac ablation procedures.”
Biosense Webster acquired Coherex Medical Inc. last November. The company is developing the Coherex WaveCrest LAAO system, which is a transcatheter-delivered, permanent LAA occluder. The WaveCrest received CE mark approval in September 2013.
New Guidelines for LAAO Devices
Last summer, the ACC, Heart Rhythm Society (HRS) and Society for Cardiovascular Angiography and Interventions (SCAI) released a new overview on the implantation of LAAO devices. As new devices are developed, it is anticipated that the use of LAAO technologies in clinical practice will expand. The authors of the paper urged that the new technology should be disseminated thoughtfully, with emphasis on team-based care and the collection of the necessary data in longitudinal registries to determine ideal patient selection, effectiveness and safety. It will also be necessary to develop and implement new guidelines, expert consensus statements, requirements for training, operator credentialing and institutional policies.
Ongoing LAA Clinical Trials
A clinical trial presented at EuroPCR 2016 in May compared the use of LAAO to standard medical treatment, using the St. Jude Amplatzer Cardiac Plug (ACP) and Amplatzer Amulet devices. Researchers assessed the impact of LAAO in patients with AF and intracerebral hemorrhage (ICH) versus patients who received standard medical therapy. The study found that patients with AF and a prior ICH who were treated with an LAAO device had a lower risk of ischemic stroke, major bleeding and all-cause mortality compared to patients treated with standard care, suggesting that LAAO offers a major clinical benefit. The FDA gave approval for a pivotal trial for the Amulet LAA occluder in September 2016.
“Patients with atrial fibrillation and previous intracerebral hemorrhage have an increased risk of ischemic stroke or repeated hemorrhage,” said Jens Erik Nielsen-Kudsk, M.D., DMSc, an associate professor of cardiologic medicine at Aarhus University, Denmark, and the study’s lead author. “The data from this latest clinical assessment further shows that having a device like the Amplatzer LAA occlusion device implanted in these patients’ hearts offers them additional opportunity for improved cardiac health.”
The first patients were enrolled in March for the aMAZE Trial to test the use of combined catheter ablation with the use of SentraHeart’s Lariat surgical LAA occluder. The device uses a lasso-like stitch to externally tie off the LAA. The LAA may also be a source of abnormal electrical activity that triggers AF. The trial will evaluate whether the combination of the two treatment approaches may treat persistent AF more effectively than catheter ablation alone. It will include 175 patients enrolled at 15 U.S. sites.
In a recently published study, the Lariat used as an adjunct to ablation in patients with persistent or long-standing persistent AF showed a 65 percent freedom from AF at one year compared with 39 percent in patients with ablation alone. Other studies have demonstrated that the device not only closes the LAA mechanically, but can also isolate electrical activity within the LAA — a known trigger for AF, shown to contribute abnormal electrical activity in a significant percentage of patients.
AtriCure Inc. announced in February that the first patient was enrolled in its ATLAS clinical study. This observational study explores the use of the AtriClip device to decrease complications associated with post-operative atrial fibrillation (POAF) by targeting specific cardiac surgery patient populations at the highest risk of developing POAF. Postoperative AF occurs in up to 30 percent of patients undergoing cardiac surgery. Research has shown that specific risk factors predict patients at greatest risk. POAF is associated with increased complications, increased reoperations and longer hospital length of stay. The ATLAS study will compare the clinical impact of patients at highest risk of developing POAF to two randomized treatment arms: surgical LAAO (using AtriClip LAA Exclusion Systems) and patients with POAF and no surgical LAA exclusion. In addition, the study will evaluate healthcare resource utilization between the two groups.
“Clinical equipoise exists between effective LAA exclusion at the time of cardiac surgery versus prophylactic anti-coagulation of POAF in patients at elevated risk of major bleeding.” said Basel Ramlawi, M.D., chairman of the Heart and Vascular Center, Valley Health System/Winchester Medical Center, Winchester, Va. “The ATLAS trial has the potential to directly impact clinical practice for hundreds of thousands of cardiac surgical patients by answering this question.”
References:







 July 31, 2024
July 31, 2024 









