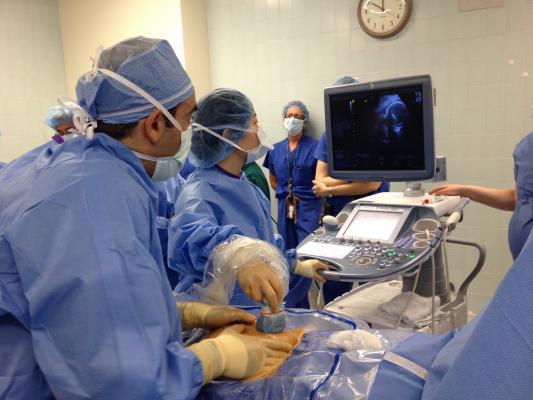
October 10, 2013 — A mother and her 25-week-old fetus are doing well after a team of physicians performed a successful in utero cardiac interventional procedure on the fetus at California Hospital Assn. (CHA) Hollywood Presbyterian Medical Center.
The minimally invasive procedure, known as a fetal aortic valvuloplasty, was a first for a Southern California hospital. Doctors succeeded in using a tiny balloon to open the fetus’s narrow aortic valve in order to increase blood flow to the body, improve left heart function and promote normal left heart growth during the critical third trimester growing stage.
Ramen Chmait, M.D., and Frank Ing, M.D., performed the procedure on Sept. 25 at Children’s Hospital Los Angeles. Collaborating for the first time, both physicians had performed the delicate fetal procedure before at hospitals outside of California.
“The surgery went beautifully; we were able to open up the aortic valve,” said Ing. “Fetal cardiac intervention is a relatively new field and requires expertise, commitment and collaboration among four specialty areas: pediatric interventional cardiology, fetal echocardiography, maternal-fetal-medicine and anesthesia.”
The patient, a 28-year-old Sylmar, Calif., mother of two, was 25 weeks pregnant at the time of procedure, which took a total of three hours and involved a team of 12, including physicians and support staff. Much of that time was devoted to Chmait’s maneuvering the fetus into position by manually adjusting the mother’s abdomen. With the assistance of a detailed ultrasound imaging by Children’s Hospital fetal cardiologist Jay Pruetz, M.D., Chmait inserted a special needle into the womb, through the fetal chest and into the peanut-sized heart’s left ventricle, positioning it below the aortic valve. As Chmait steadied the needle, Ing used Chmait’s hand as a platform for his hand to thread a hair-thin wire through the needle and out the tip, positioning the wire across the fetus’s aortic valve. The wire was then used as a rail for Ing to maneuver a tiny balloon-tipped catheter into position across the valve. Ing carefully inflated the balloon, which inflated to open the valve and increase blood flow into the aorta. The balloon, wire and needle were then removed.
The time from actual needle insertion to the balloon opening took roughly 15 minutes, Ing says. The mother was sedated during the procedure.
The fetal aortic valvuloplasty procedure has been completed more than 100 times in the United States., with most taking place at Boston Children’s Hospital. Only a handful of hospitals west of the Mississippi River have performed the procedure.
For more information: www.losangelesfetaltherapy.org, www.hollywoodpresbyterian.com


 December 24, 2025
December 24, 2025 









