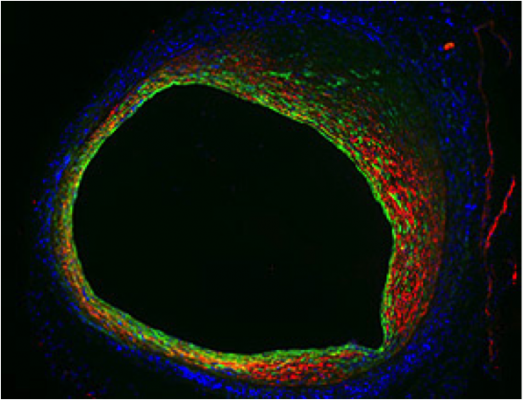
Endothelial cells not only form the inner lining of a blood vessel, but also contribute to blood vessel narrowing as shown in this mouse vein graft model. Endothelial cells (green) lose their typical morphology and become more like smooth muscle cells (red). This change in cellular properties indicates that endothelial-to-mesenchymal transition (EndMT) is operative in vein graft stenosis.
March 13, 2014 — National Institutes of Health researchers have identified a biological pathway that contributes to the high rate of vein graft failure following bypass surgery. Using mouse models of bypass surgery, they showed that excess signaling via the transforming growth factor beta (TGF-Beta) family causes the inner walls of the vein become too thick. This slows down or sometimes even blocks the blood flow that the graft was intended to restore. Inhibition of the TGF-B signaling pathway reduced overgrowth in the grafted veins.
Manfred Boehm, M.D., chief of the Laboratory of Cardiovascular Regenerative Medicine at NIH’s National Heart, Lung, and Blood Institute, led the team. It identified similar properties in samples of clogged human vein grafts, suggesting that select drugs might be used in reducing vein graft failure in humans.
This study was published March 12 in Science Translational Medicine.
Bypass surgery to restore blood flow hindered by clogged arteries is a common procedure in the United States. The great saphenous vein, which is the large vein running up the length of the leg, often is used as the bypass conduit due to its size and the ease of removing a small segment. After grafting, the implanted vein remodels to become more arterial, as veins have thinner walls than arteries and can handle less blood pressure. However, the remodeling can go awry and the vein can become too thick, resulting in a recurrence of clogged blood flow. About 40 percent of vein grafts experience such a failure within 18 months of the operation.
Boehm and his colleagues examined veins from mouse models of bypass surgery, and discovered that a process known as an endothelial-to-mesenchymal transition, or EndoMT, causes the inside of the vein to over-thicken. During EndoMT, many of the endothelial cells that line the inner surface of the vein proliferate and convert into more fibrous and muscle-like cells. These mesenchymal cells begin to accumulate on the inner wall, narrowing the vessel.
This process was triggered by TGF-Beta, a secreted protein that controls the proliferation and maturation of a host of cell types; the researchers found that TGF-Beta becomes highly expressed just a few hours after graft surgery, indicating the remodeling starts fairly quickly.
Boehm’s team also looked at human veins taken from failed bypass operations, and found corroborating evidence for a role for EndoMT in human graft failure. In short term grafts (less than one year), many of the cells inside the human veins displayed both endothelial and mesenchymal cell characteristics. In long-term grafts (more than six years) the cells on the inner wall were primarily mesenchymal in nature.
“This study shows for the first time that endothelial cells in the vein directly contribute to blood vessel narrowing following a vein graft,” Boehm said. “Now that we better understand the mechanism that causes the abnormal thickening, we can look for therapeutic strategies to attenuate it, and reduce the number bypass reoperations we need to perform each year.”
Boehm cited the high-blood pressure drug Losartan, which can inhibit TGF-Beta, as one possible treatment strategy, though more proof of concept studies are needed before any clinical studies can commence.
In addition to Boehm’s lab at NHLBI, other contributors to this study included the Medical College of Wisconsin, Milwaukee; Barts and the London NHS Trust, London, UK; Cairo University, Egypt; University of Glasgow, Scotland, UK; Tubingen University, Germany; CVPath Institute Inc., Gaithersburg, Md. and the Mount Sinai School of Medicine, New York City.
For more information: www.nih.gov


 February 06, 2026
February 06, 2026 









