
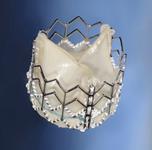
Abbotts MitraClip device is a transcatheter repair system to reduce mitral valve regurgitation.
The FDA’s approval of the Melody valve is widely viewed as a positive sign that may help other transcatheter valves to follow.
“This is really a bellwether in terms of the FDA regulator pathway that hopefully other manufacturers will be able to follow,” said Tom Jones, M.D., director of Seattle Children’s Hospital cardiac catheterization laboratory professor of pediatrics and medicine at the University of Washington School of Medicine, and an investigator with the Melody trial. “It has created a pathway for approval for other [transcatheter valve] devices.”
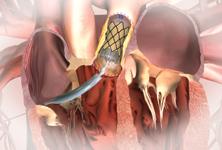
The next transcatheter valve that is likely to gain FDA clearance is the Edward’s Lifesciences Sapien transcatheter aortic valve. If approved, the valve will have a much more significant impact on the transcatheter market than a pulmonary valve. Melody will be used for about 3,000 valve replacements annually in the United States, while mitral and aortic valve replacements total about 99,000.
Edwards said at TCT 2009 it hopes the FDA may review Sapien for market clearance in 2011. In the U.S., Edwards has completed a feasibility study and the PARTNER Trial, which is the pivotal trial that will be utilized for FDA submission. The company hopes to begin its PARTNER II U.S. trial in the fourth quarter of 2009, which will target high-risk patients using the the Edwards Sapien XT valve. Both the Sapien and the Sapien XT are cleared for use overseas.
“Thousands of patients have been treated so far, and the outcomes have exceeded our expectations,” said Michael A. Mussallem, Edwards Lifesciences’ chairman and CEO during TCT 2009. In a recent update on the company’s earnings, he said Edwards more than doubled its overseas sales of the Sapien, totaling $112 million.
The Sapien uses a balloon-expandable stainless steel stent frame and a bovine tissue valve. The Edwards Sapien XT valve uses a cobalt chromium frame.
Another transcatheter valve that is expected to seek FDA clearance is the CoreValve aortic valve, which Medtronic purchased in late 2009. The valve was cleared for use in Europe in 2007. Medtronic expects to begin the valve’s U.S. trial this summer. It features a porcine pericardium valve mounted in a self-expanding stent frame. Medtronic said it is already working on an improved second-generation device to replace CoreValve.
In 2009 Medtronic also purchased Ventor, an Israeli company developing the Embracer aortic transcatheter valve, which is currently in trials seeking CE mark in Europe. It is placed using a minimally invasive procedure through the apex of the heart. The shape of the device grips the native valve leaflets with loop wires to ensure a good fit and anatomically correct alignment. It uses a bovine tissue valve.
Another player in the U.S. transcatheter valve market is Abbott. Its pivotal U.S. trial, EVEREST II (Endovascular Valve Edge-to-Edge REpair STudy), is underway for the MitraClip device. The transcatheter repair system reduces mitral valve regurgitation. The randomized clinical trial of 279 patients is evaluating the safety and efficacy of MitraClip compared to open heart surgery. The latest trial data will be released at ACC 2010. Abbott anticipates FDA approval in 2011.
The device secures the leaflets of the mitral valve together with a clip, which preserves surgical options in case a patient later needs a more extensive repair. The MitraClip is the only catheter-based mitral valve repair technology to treat regurgitation on the market in Europe.


 December 24, 2025
December 24, 2025 









