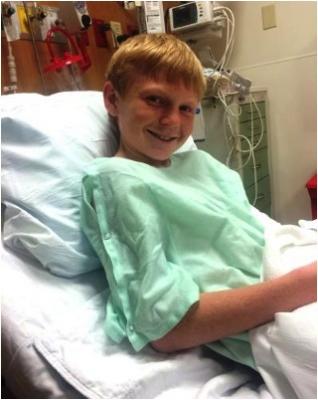
December 15, 2014 — Doctors at St. Joseph’s Children’s Hospital of Tampa implanted a Melody transcatheter pulmonary valve in a 13-year-old patient in October, marking the 50th time the hospital has given the device to a patient.
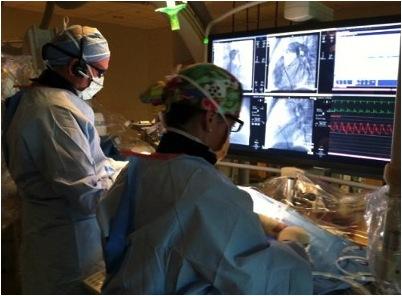
Thirteen-year-old Cyrus Yarley was born with multiple heart defects and underwent open-heart surgery at just 1 week old then again at age 8. His mother, Valorie Daniels, knew the day would come when Cyrus would need to have his pulmonary valve replaced, and had anticipated another open-heart surgery for the past five years. Use of the Melody valve, however, precluded the need for an additional incision. After inserting a catheter into a vein in the upper leg, pediatric cardiologist Jeremy Ringewald, M.D., guided the Melody valve up to Cyrus' heart and replaced his current dysfunctional pulmonary valve.
The Melody valve from Medtronic replaces narrow or leaky pulmonary valve conduits in children or adults who have previously undergone congenital heart surgery. St. Joseph's Children's Hospital is the highest-volume Melody valve implanting center in Florida and one of the busiest in the United States.
"Children born with blocked or leaky heart valves usually undergo multiple open-heart surgeries before reaching adulthood to replace conduits that have worn out or that they have outgrown," said Ringewald. "This groundbreaking technology can markedly prolong the time between surgeries for many patients, with the goal of maximizing heart health and minimizing invasiveness."
According to Ringewald, the Melody valve procedure is typically performed under general anesthesia in less than four hours. Unlike open-heart surgical procedures, where the hospital stay is approximately a week, patients are usually discharged from the hospital within 24 hours.
While Melody valve therapy cannot be used in all cases of abnormal pulmonary valve function, it can give doctors an alternative to repeated traditional open-heart procedures, offering children and adults who may not be able to tolerate another open-heart surgery hope for the future.
For more information: www.medtronic.com


 December 24, 2025
December 24, 2025 









