
October 14, 2015 — Claret Medical, an innovator in cardiovascular cerebral protection, Data presented this week at Transcatheter Cardiovascular Therapeutics (TCT) 2015 showed the first definitive cognitive benefit from use of a cardiovascular cerebral protection system during transcatheter aortic valve replacement (TAVR) procedures.
Data from the MISTRAL-C study show that patients who received a Claret Medical Sentinel Cerebral Protection System (CPS) during TAVR faired better than unprotected patients. Unprotected patients had a statistically significant (p=0.017) worsening in cognition when compared to Sentinel-protected patients at five days post-TAVR, when assessed using the Mini Mental State Exam (MMSE).
MISTRAL-C also validates findings from the landmark CLEAN-TAVI study that showed the use of a Claret Medical cerebral protection system reduced the number and volume of brain lesions in TAVR patients.[1] MISTRAL-C showed a 52 percent reduction in the median total new lesion volume at five days post-procedure as assessed using highly sensitive 3.0 Tesla brain MRI. None of the protected patients had National Institute of Health Stroke Scale (NIHSS) deterioration at five days post-procedure, while 5 percent of unprotected patients showed deterioration.
MISTRAL-C studied 65 patients enrolled at four centers in the Netherlands that underwent TAVR using both self-expanding and balloon-expandable valves, with and without the use of the Sentinel CPS. The study compared the median total new lesion volume as detected by DW-MRI at five days post-procedure to pre-procedural baseline cerebral scans. It also included a detailed neurological and cognitive assessment of patients five days post-procedure.
“What is clear in MISTRAL-C is that patients not protected by the Sentinel CPS showed significant cognitive decline post-procedure when compared to those protected by the Sentinel CPS. Protected patients also showed a robust reduction in the number and volume of new cerebral lesions at five days,” said Principal Investigator Nicholas van Mieghem, M.D., Thoraxcenter, Erasmus Medical Center, Rotterdam.
Also presented at TCT was a post hoc, 3-D voxel-wise analysis of CLEAN-TAVI DW-MRI images illustrating that only two percent of the left posterior region of the brain remains fully unprotected when a Claret Medical cerebral protection system is used. The data were presented by Axel Linke, M.D., professor of medicine, Department of Internal Medicine/Cardiology, University of Leipzig, Germany.
“Post-hoc neuroimaging and neurological analysis is providing a more complete, and troubling, picture of the brain post-TAVR,” Linke said. “Evidence is building that more sustained damage than we previously realized may be created when we don’t protect the brain from embolic debris. The use of cerebral protection, however, has now been shown to effectively shield the brain from potentially dangerous debris showers caused by the TAVR procedure.”
Claret Medical Chief Executive Officer Azin Parhizgar, Ph.D., said by incorporation of 3.0T MRI analysis at baseline in clinical studies, CLEAN-TAVI showed nearly 40 percent of patients present with pre-existing lesions, and 17 percent of those with volume greater than 100 mm.[2] Utilizing the more sensitive 3.0T MRI enables an accurate way to quantify the number and volume of all new lesions caused by TAVR.
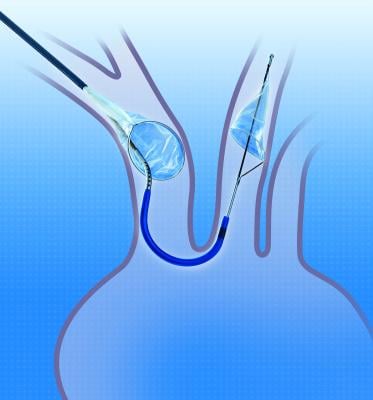
The Sentinel CPS received the CE mark in 2013 and now, more than 2,000 commercial cases have been performed using Claret Medical’s cerebral protection technology. The Sentinel CPS is also currently being studied in the United States and Europe as part of the SENTINEL pivotal trial, the first FDA-approved trial of a cerebral protection system and the largest randomized trial in the field to date.
For more information: www.claretmedical.com
References:
1. Linke A. “CLEAN-TAVI: A Prospective, Randomized Trial of Cerebral Embolic Protection in High Risk Patients with Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement.” Transcatheter Cardiovascular Therapeutics (TCT). 2014
2. van Mieghem N et al. “Histopathology of Embolic Debris Captured During Transcatheter Aortic Valve Replacement.” Circulation. 2013;127:2194-2201


 October 31, 2025
October 31, 2025 









