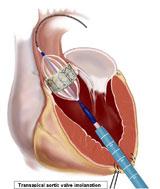
The Cardiopulmonary Research Science and Technology Institute (CRSTI) in Dallas, Texas, created two hybrid operating rooms (ORs) specifically for participation in the PARTNER trial for Edwards Sapien transcatheter aortic valve. The center has implanted more than 200 Sapien valves since it started in the trial in 2006.
Angela Riley, RT, CRSTI executive director, said the cost of transcatheter aortic valve implantation (TAVI) and the build out of the hybrid ORs will be prohibitive for most hospitals and the return on investment (ROI) is low. However, she said patients are drawn to facilities offering TAVI.
She said patient selection for TAVI is critical and many patients do not qualify. Her center screens four patients to get one who is suitable for TAVI. Most of those turned away undergo surgical valve replacement. While this is not a good ratio to base return on investment (ROI) for building a TAVI program, Riley said the real returns have come from tripling surgical referrals.
A better strategy to sell the costs of a TAVI program to hospital administrators is not to base ROI on TAVI procedures, but on the increased surgical valve repair volume from the referrals. “Even if patients do not qualify, they will usually stay with your hospital for other treatments,” she explained.


 December 24, 2025
December 24, 2025 









