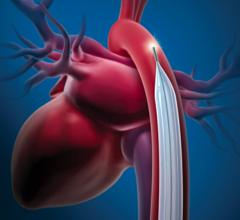
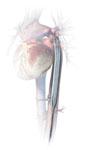
The TandemHeart system takes oxygenated blood from the left atrium using a pump strapped to the patients thigh and flows it into the femoral artery. The device requires a 17 Fr. transseptal puncture.
Intra-aortic balloon pumps (IABPs), introduced in the 1970s, have been considered the standard of care for short-term circulatory support for more than two decades. However, there has been a growing trend over the past few years toward percutaneous ventricular assist devices (VADs), which some cardiologists say have some big advantages over IABPs.
There are many VADs on the market used as a bridge to transplant and destination therapy for class IV heart failure patients. Only three VADS with FDA clearance qualify as percutaneous circulatory support devices suitable for quick set up and use in the cath lab – Cardiac Assist’s TandemHeart and Abiomed’s Impella 2.5 and 5.0 devices. There are four FDA-cleared IABPs – Datascope’s CS100 and CS300, Abiomed’s iPulse and Arrow International’s AutoCAT 2 WAVE.
These VADs and IABPs are generally used with patients who have ejection fractions below 35 percent. This includes patients with class III and IV heart failure, cardiogenic shock, or those undergoing high-risk percutaneous interventions where the device is used as a safety net. Neither TandemHeart nor Impella have a specific FDA indication for cardiogenic shock, but IABPs do.
Currently Available IABPs
Abiomed estimates about 160,000 IABPs are used globally each year, with about 110,000 of those used in U.S. procedures. IABPs by far outpace the usage of percutaneous VADS, which have been used in fewer than 3,000 patients in the United States.
These pumps use a balloon catheter inserted into the aorta that counterpulsates in sync with heart rhythms based on ECG.
Datascope Corp., which introduced the first IABP in 1970, produces two IABPs with FDA clearance, the CS100 and the CS300. Both devices use computerized sensors to automatically adapt to rate and rhythm changes. The CS300 uses a fiber-optic catheter system that automatically calibrates in the patient after insertion and automatically recalibrates in vivo every two hours, or sooner should patient or environmental conditions change. Datascope said the CS100 offers consistent support for patients with PVCs and arrhythmias by automatically evaluating and selecting the optimal trigger source and lead. It responds to changes in signal quality by selecting a new trigger source.
In 2008 Datascope released the Sensation 7 Fr., which the company says is the smallest IAB catheter available.
Arrow International offers the AutoCAT 2 WAVE IABP, which the company said can provide support to the most unstable patients with tachyarrhythmias and variable pulse pressures. Arrow said its system can maintain a 1:1 assist.
The Arrow system uses fiber-optic technology, which uses a physiologic timing algorithm called WAVE to adjust automatically. The system’s AutoPilot Operation is designed to make automatic timing and triggering adjustments to optimize counterpulsation support. Early inflation of the IAB within the cardiac cycle has been shown to severely compromise left ventricular function. When used with the system’s FiberOptix IAB catheter, the AutoCAT 2 WAVE system calculates patient aortic flow on a beat-to-beat basis, instantaneously determining the aortic valve closure point for that beat. Aortic flow timing sets inflation beat to beat, automatically, matching inflation of the balloon to within 12 msec. of the time of aortic valve closure even with severe arrhythmias.
During arrhythmias, automatic R-wave deflation, coupled with aortic flow timing, delivers the closed-loop IABP timing system.
Abiomed offers the iPulse control console, which enables use of Abiomed’s IABP. The console also will control Abiomed’s BVS 5000 and AB5000 surgical VADS.
IABP vs. VADS
Samin Sharma, M.D., director of interventional cardiology and cardiac cath lab, professor of medicine, Mount Sinai Medical Center, New York, said at TCT 2009 that IABPs see wider usage because they are less expensive than Impella or TandemHeart. He said they are also simple to use and most cath labs already have them in stock. However, in some cases he said IABPs do not provide the level of circulatory support that is needed. “It also requires time and you have to puncture vessels with a 21 French catheter,” he said.
Other issues with IABPs include increased aortic wall tension, the balloon pump forces blood both forward and back toward the heart, it does not unload the left ventricle (LV) and IABPs can not be used in patients with aortic valve regurgitation, said William O’Neill, M.D., FACC, FSCAI, professor and former dean of clinical affairs at the University of Miami, division of cardiology, Miami, Fla., during TCT 2009. He said percutaneous VADS also offer more support than IABPs.
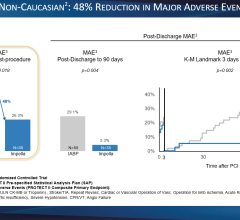
Sometimes IABPs do not provide enough support and he pointed to the data released at TCT 2009 from USpella, the first U.S. multicenter registry of Impella 2.5 patients to evaluate the safety and feasibility of LV support during high-risk PCI and treatment of acute myocardial infarction. Of the 181 patients who went into cardiogenic shock and received support from Impella 2.5, the device was used only after conventional therapies failed. This included 88 percent getting the device after emergent revascularization, 88 percent after high-dose inotropes, and 68 percent after IABP therapy failed to deliver the needed support. “A lot of these patients are surviving now, where they would not have survived on the balloon pump,” Dr. O’Neill said.
In addition to forward-only flow and more flow support than IABPs, transcatheter VADs can be used without a stead heart rhythm, said John Lasala, M.D., Ph.D., director of invasive cardiology, professor of medicine, Washington School of Medicine, Barnes-Jewish Hospital, St. Louis. He said IABPs usually are used with inotropes to keep the heart at a steady rhythm.
“The flow increase is relatively modest,” Dr. Lasala said of IABPs. However, with the Impella 2.5, he said there is a better blood flow increase and the device directly helps unload the LV. “Impella can be enough to reverse problems and help patients recover, or respond better to inotropes than without support,” he said.
Jose Henriques, M.D., Ph.D., cath lab director, Academic Medical Center, University of Amsterdam spoke at an Abiomed evening symposium at TCT 2009 and pointed to several clinical studies where Impella out-performed IABPs with better outcomes, including the ongoing PROTECT II and RECOVER II clinical studies.
There are no comparable studies between TandemHeart vs. IABPs, but users say the TandemHeart provides more hemodynamic support than balloon pumps.
Percutaneous VADS
The Impella 2.5 LP is the smallest circulatory support device on the market, making it easier to insert and use during cath lab procedures. The device, cleared by the FDA in June 2008, uses a 13 Fr. introducer sheath in the femoral artery. The catheter is fed into the LV and uses a small impeller inside the catheter to draw blood from the LV into the aorta. Physicians who use the device say it provides more support than an IABP, but its 2.5 lpm of flow may not be enough to help patients with severely compromised heart function.
In April 2009 the FDA cleared the Impella 5.0, offering a flow rate of 5 lpm. The pump is housed in a 9 Fr. catheter. There is currently no introducer sheath made for the device, so Abiomed said its use requires a surgical cut down to gain a 21 Fr. access point in the femoral artery.
Cardiac Assist’s TandemHeart PVTA System was FDA cleared in 2003 and has been used in more patients than Impella. It offers a high net flow rate of a up to 5 lpm in the cath lab or up to 8 lpm in the operating room. The higher rate in the OR is due to the ability of using a direct 24 Fr. cannula placed in the heart during open procedures. Its transcatheter deployment uses two cannulas using a 17 Fr. arterial catheter introducer sheath and a 21 Fr. venous introducer sheath. The device requires a 17 Fr. transseptal puncture in the heart to insert a cannula from the femoral vein into the left atrium. Oxygenated blood is drawn from the left atrium using a pump strapped to the patients thigh and flows it into the an arterial cannula inserted in the patient’s femoral artery. The company says surgical cut downs are needed for cannula insertion in about 5 percent of patients. CardiacAssist says the advantage of TandemHeart is it can still pump blood and help a patient without a pulse, where as Impella requires some native LV ejection. The TandemHeart is able to still pump blood without a pulse because it bypasses the LV.
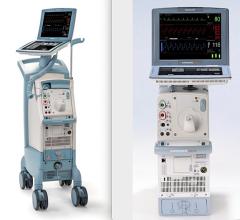
Two console options are available for the TandemHeart, including a cart-mounted version and an IV pole-mounted version.
TandemHeart and Impella 2.5 and 5.0 are all FDA cleared for circulatory support of periods up to six hours.
Abiomed is developing the Impella RP 2.5, which is designed for right ventricle support. The company is also working on a miniature pediatric version of the Impella that will have a flow of 1.5 lpm. No timeline has been set for the release of either device.
CardiacAssist is developing the TandemTot circulatory support device for infants and neonates. The company said the device will have a flow rate of 1-1.5 lpm. No timeline has been set for the product’s release.



 August 14, 2023
August 14, 2023