October 26, 2022 — Abiomed (Nasdaq: ABMD) announces a new program to address healthcare disparities in underserved communities, as new data provides an example of how better access to Impella heart pumps can improve health equity for non-Caucasian cardiovascular patients. Data from a subgroup analysis of 93 non-Caucasian high-risk PCI patients enrolled in the PROTECT II Randomized Controlled Trial (RCT) finds those who were treated with Abiomed’s Impella heart pump had significantly improved clinical outcomes compared to those who were treated with the intra-aortic balloon pump (IABP).
Specifically, the analysis found, when compared to patients who received IABP, Impella patients experienced:
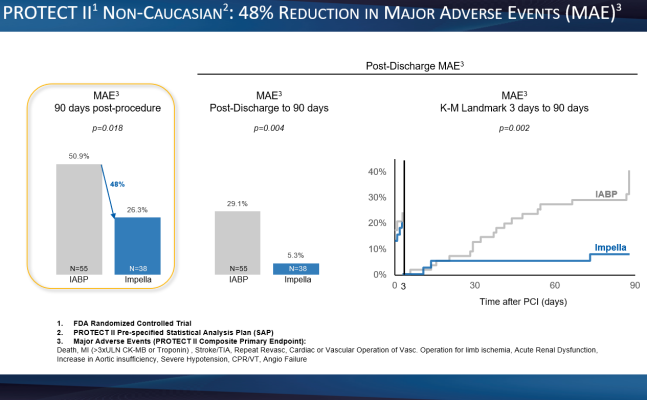
- A 48% reduction in major adverse events (MAE), the primary endpoint of the PROTECT II RCT, out to 90 days post-procedure. (p=0.018) (see figure 1)
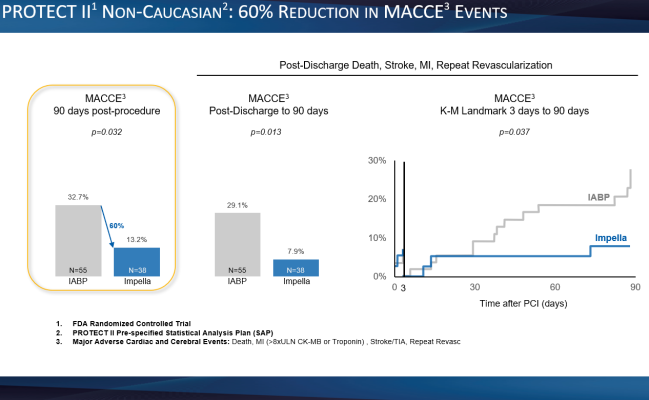
- A 60% reduction in major adverse cardiac and cerebral events (MACCE) out to 90 days post-procedure. (p=0.032) (see figure 2)
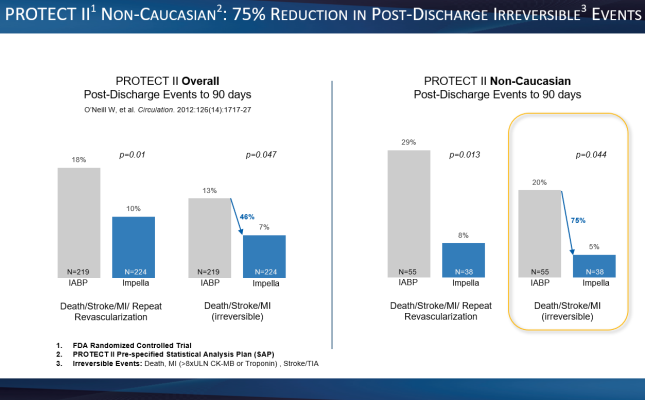
- A 75% reduction in irreversible events (death, stroke, myocardial infarction) from discharge to 90 days post-procedure. (p=0.044) (see figure 3)
The analysis of this subset population was pre-specified in the statistical analysis plan for the MAE primary endpoint of the PROTECT II RCT. Patients in the analysis were included in Abiomed’s regulatory submission to the U.S. Food and Drug Administration (FDA) that resulted in the FDA granting Impella approval as safe and effective for high-risk PCI.
“This data about the benefit of Impella-supported procedures for non-Caucasian patients is highly compelling and should be used to inform physicians’ clinical decision-making when treating non-Caucasian patients who have heart disease,” said William O’Neill, MD, medical director of the Center for Structural Heart Disease at Henry Ford Health and the principal investigator of the PROTECT II RCT.
As a result of this subgroup analysis, and the abundance of other clinical data that demonstrates the benefits of Impella heart pumps in high-risk PCI, cardiogenic shock and right heart failure, Abiomed is establishing a patient assistance program to help reduce treatment disparities in the healthcare system. The W. Gerald Austen Disparities in Healthcare Initiative will provide assistance to hospitals in the United States and the Bahamas that use Impella best practice protocols to treat patients who are on Medicaid or who are underinsured. It is an expansion of Abiomed’s existing diversity initiatives.
“Systematic and social factors create disparities in the treatment options available to heart disease patients who are candidates for a PCI procedure. This program is a step toward righting these disparities and improving healthcare in underserved communities by helping all patients receive appropriate care when they are in cardiogenic shock, right heart failure, or in need of a Protected PCI,” said Myron Rolle, MD, global neurosurgery fellow at Massachusetts General Hospital, chair of the Caribbean Neurosurgery Foundation and a member of the Abiomed Board of Directors. “I am particularly excited this initiative will help in the Bahamas, an island nation of more than 90% African descent with ischemic heart disease as the number one cause of death.”
The program is named in memory of Dr. Gerald Austen, the former chief of surgical services at Massachusetts General Hospital (MGH) and a 37-year member of the Abiomed Board of Directors. Dr. Austen was also the founding president and CEO of the Massachusetts General Physicians Organization, the first physician elected to the MGH Board of Trustees and a founder of the Partners HealthCare system.
“Dr. Austen was an inspiration to the field of cardiology, Mass General Hospital, Abiomed and all those around him. His life was one of service to patients, medicine and his family. Abiomed will honor Dr. Austen with this healthcare disparity program as a service to patients and communities in need, to which he dedicated his life’s work,” said Michael R. Minogue, Abiomed Chairman, President and Chief Executive Officer.
For more information about the W. Gerald Austen Disparities in Healthcare Initiative, please visit www.abiomed.com/dih-initiative.


 January 05, 2026
January 05, 2026 









