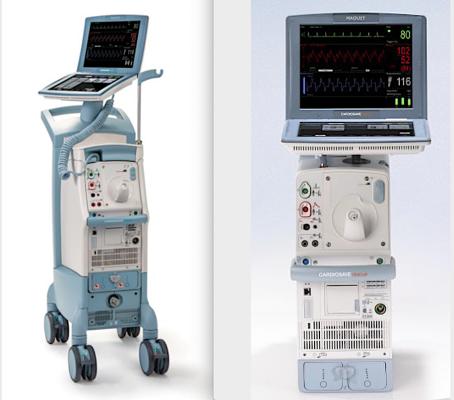
Cardiosave Hybrid Intra-Aortic Balloon Pump, left, and the Cardiosave Rescue Intra-Aortic Balloon Pump. Both systems have a recall because of reports that the battery can fail.
November 2, 2021 — Datascope/Getinge/Maquet is recalling its Cardiosave Hybrid/Rescue Intra-Aortic Balloon Pumps (IABP) because of reports the battery packs can fail. The U.S. Food and Drug Administration sent out a notice this week that this is a Class I Recall because of the risk of serious injury or death.
The CardioSave IABPs are cardiac assist hemodynamic support devices used with patients undergoing cardiac and non-cardiac surgery, and to treat adult patients who have acute coronary syndrome or complications of heart failure.
The company recalled the product due to the risk of the battery failing and having a shortened run-time due to substandard batteries not meeting performance specifications being released to customers. The vendor said these batteries may cause the device to stop working when operated by battery only.
If a patient requires life-supporting therapy with an IABP and the device does not work or if therapy is stopped during use due to battery failure, the patient will be at risk of serious injury, including death. Both Cardiosave Hybrid and Rescue IABP monitors display battery life to the user, prompting intervention with low battery alarms when alternative power sources are indicated.
Datascope/Getting/Maquet reported receiving six complaints about this issue, but there have not been any reports of injuries or deaths related to this issue. However, there is a potential for underreporting since the end user reporting a failed battery or short battery-run time cannot be aware that they originally received a substandard battery, the company said.
For more information read the recall notice.
List of all recalled serial numbers.
On Sept. 21, 2021, Datascope/Getinge/Maquet sent an “Urgent Medical Device Removal" letter to customers requesting that they:
• Examine inventory immediately to determine if there are any Cardiosave Li-Ion battery packs with part number/REF number 0146-00-0097 and with serial numbers matching those listed at the top of the “Urgent Medical Device Removal" letter.
• Replace any affected battery with an unaffected battery and remove affected product from areas of use.
• Replace any affected battery with an unaffected battery and remove affected product from areas of use.
• Should you have the affected product, you are eligible for credit or a replacement at no cost to the facility upon receipt of the response form attached to the “Urgent Medical Device Removal" letter.
• To get the free replacement battery, provide your shipping information and your acknowledgment on page 4 that the defective battery will be disposed once you receive the replacement battery pack.
• Dispose of affected batteries properly in accordance with local statutes and the labeling on the battery pack.
• Forward this information to all current and potential Cardiosave Hybrid and Cardiosave Rescue IABP users within your hospital or facility.
• Whether you have affected product or not, please complete and sign the "Urgent Medical Device Removal" response form attached to the letter to acknowledge that they received this notification and disposed properly of the affected product.
• Return the completed form to Datascope/Getinge/Maquet by e-mailing a scanned copy to [email protected] or by FAX to (877)-446-3360.
The FDA said health professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety reporting system.
Find more information on IABPs
Find more cath lab technology news


 October 23, 2023
October 23, 2023