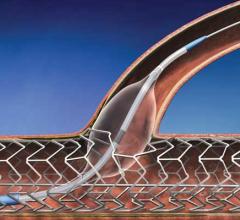
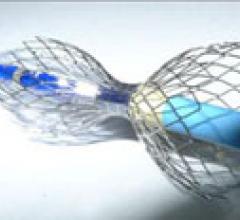
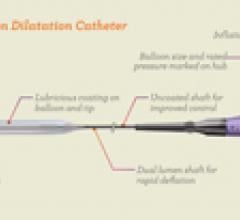
June 21, 2012 — TriReme Medical Inc. announced today it received U.S. Food and Drug Administration (FDA) clearance for ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
June 13, 2012 — Physicians presented at EuroPCR 2012 the results of two multicenter, randomized controlled trials, the ...
May 30, 2012 — Boston Scientific Corp. announces the U.S. Food and Drug Administration has cleared an expanded use ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 23, 2012 - TriReme Medical Inc. announced approval by the U.S. Food and Drug Administration (FDA) of the Glider PTCA ...
May 4, 2012 - Angioslide Ltd., a provider of embolic capture angioplasty solutions, announced that it has received U.S ...
May 4, 2012 - A new balloon catheter system could advance the endovascular approach to treating obstructed arteries in ...
April 24, 2012 - Boston Scientific Corp. announces CE mark and European market launch of the Emerge PTCA Dilatation ...
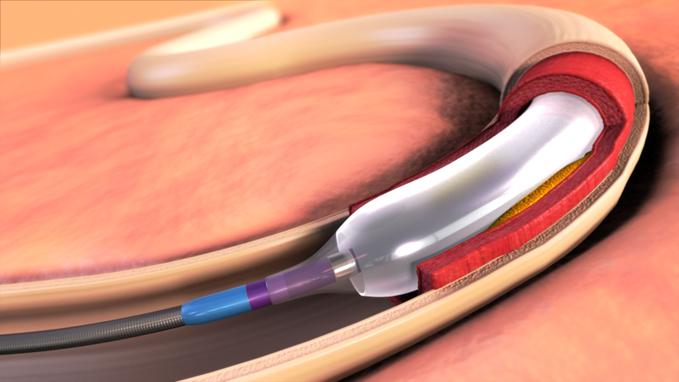
April 17, 2012 –– Expanding its commitment to developing innovative treatments for cardiovascular disease and the ...
March 20, 2012 - MarBlue Medical announced the global product launch of its CE-marked drug-eluting balloon (DEB) Protege ...
January 25, 2012 — Physicians at University Hospitals (UH) Case Medical Center enrolled their first patient in LEVANT 2 ...
January 6, 2012 - C. R. Bard recently announced it acquired drug-eluting balloon maker Lutonix Inc. for $225 million ...
December 28, 2011 – Good Samaritan Hospital in Los Angeles is participating in the Levant 2 clinical trial to ...
December 15, 2011 — Neovasc Inc. announced it has received the CE mark designation for its Reducer product for the ...
December 13, 2011 – Boston Scientific announced the U.S. launch of its Charger PTA Balloon Catheter, a 0.035-inch ...
November 15, 2011 – Results from the PROFI study indicate that the use of a proximal balloon occlusion in carotid artery ...

 June 21, 2012
June 21, 2012