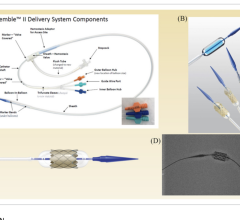
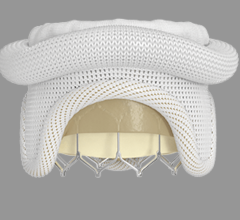
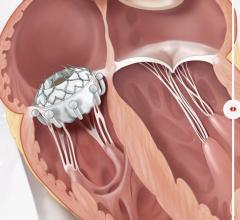
March 16, 2023 — Cardiologists at the UW Medicine Heart Institute recently performed a first-in-the-world procedure ...
Heart Valve Technology
This channel includes news and new device innovations about heart valve technologies, including the aortic valve, mitral valve, pulmonic valve, and tricuspid valve. This includes information on transcatheter valve technologies like transcatheter aortic valve replacement (TAVR, or implantation TAVI), transcatheter mitral valve repair or replacement (TMVR), transcatheter and surgical valve repairs, and surgical replacement valves. Newer devices are now being used for transcatheter tricuspid valve repair replacement (TTVR).
May 9, 2023 — Edwards Lifesciences announced new data from the COMMENCE aortic trial, demonstrating low rates of structu ...
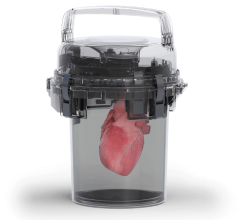
April 6, 2023 — Paragonix Technologies, Inc., a leading organ transplant company, announces new research from a multi ...
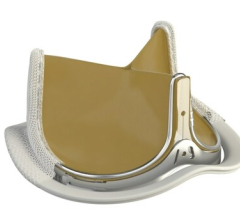
March 31, 2023 — Abbott announced that the U.S. Food and Drug Administration (FDA) has approved the company's Epic Max ...
February 28, 2023 — On Feb. 27, the U.S. Food and Drug Administration (FDA) issued a Letter to Health Care Providers to ...
January 23, 2023 — Leakage of the mitral valve due to degenerative prolapse is a common condition known as primary ...
January 6, 2023 — Patients with congenital heart diseases often suffer from obstructions in the right ventricular ...
January 2, 2023 — The U.S. Food and Drug Administration (FDA) has given the approval to market to The Edwards ...
December 29, 2022 — The Smidt Heart Institute at Cedars-Sinai was named a Mitral Valve Repair Reference Center, a ...
December 14, 2022 — Edwards Lifesciences identified the top data releases from 2022 that contributed most to shaping ...
December 13, 2022 — Edwards Lifesciences Corporation announced that one-year results on patients treated in the single ...
December 2, 2022 — Boston Scientific Corporation has announced the first results from the ACURATE neo2 Post Market ...
November 30, 2022 — Boston Scientific Corporation has announced the first results from the ACURATE neo2 Post Market ...
November 22, 2022 — CroíValve has announced the successful First in Human implants of its DUO Tricuspid Coaptation Valve ...

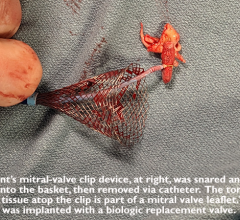
 May 16, 2023
May 16, 2023