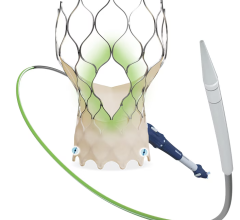
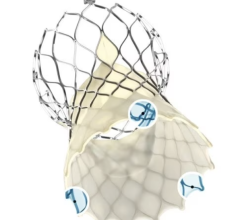
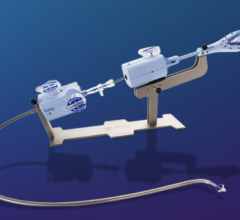
January 2, 2023 — The U.S. Food and Drug Administration (FDA) has given the approval to market to The Edwards Lifesciences PASCAL Precision Transcatheter Valve Repair System. The PASCAL Precision Transcatheter Valve Repair System is used to repair the mitral valve of the heart without the need for open-heart surgery. The device system includes a permanent implant and a delivery system. The primary components of the implant are constructed from nitinol (a nickel titanium alloy) and covered in polyethylene terephthalate (PET) cloth.
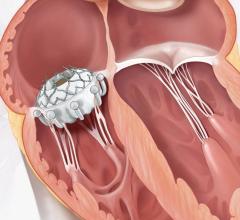
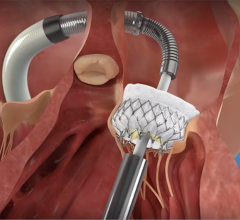
The PASCAL Precision Transcatheter Valve Repair System implant is inserted using a tube-like device called a delivery catheter through the largest vein (femoral vein) in the groin area and guided through the blood vessel to the left side of the heart. The permanent implant looks and functions like a clip. Once in the heart, the clip is used to grasp the two tissue flaps (leaflets) of the mitral valve in the heart, which is the valve that controls blood flow between the top and bottom chambers of the heart’s left side. The implant clips the two flaps together to reduce the backflow of blood through the valve (mitral regurgitation). After the clip is in place, it is disconnected from the delivery catheter and then the delivery catheter is removed from the body.
The PASCAL Precision Transcatheter Valve Repair System is intended to be used to treat patients with significant (a grade greater than or equal to 3+) mitral regurgitation caused by abnormal mitral valve leaflets and/or the area around the leaflets when open-heart surgery is considered too risky.
The PASCAL Precision Transcatheter Valve Repair System is intended to reduce the amount of blood that moves in the wrong direction through the mitral valve. As a result, the patient may have:
- fewer hospitalizations
- improved quality of life
- relief of symptoms such as tiredness or fatigue
- improved ability to exercise
A clinical study compared the results of 117 patients treated with the PASCAL Precision Transcatheter Valve Repair System with of 63 patients who were treated with a commercially available similar device. The study showed that the two devices had similar low risks for major complications at 30 days post-implant and reduction in the amount of blood flowing back through the valve (lowering mitral regurgitation grade to less than or equal to 2+) at 6 months post-implant.
The PASCAL Precision system should not be used in patients who:
- Cannot tolerate certain blood thinners during or after the procedure
- Have an untreatable allergy to nickel, titanium or X-ray contrast media
- Have an active infection of the mitral valve (endocarditis)
- Have mitral regurgitation caused by rheumatic disease
- Have evidence of blood clots in the heart or veins leading to the heart
For more information: www.fda.gov
Related PASCAL news items:
Edwards Lifesciences and Abbott Settle Transcatheter Mitral Valve Device Patent Litigation
Related Transcatheter Mitral Valve Device Content:
Developments in Transcatheter Mitral Valve Replacement
Transcatheter Tricuspid Valve Repair Clip Device Cleared in Europe
New Study Looks at MitraClip use in Moderate Surgical Risk Patients
Edwards Pascal Tricuspid Valve Transcatheter Repair System Approved in Europe
Edwards Announces Research Milestones for Pascal Transcatheter Mitral Valve Program


 July 08, 2024
July 08, 2024