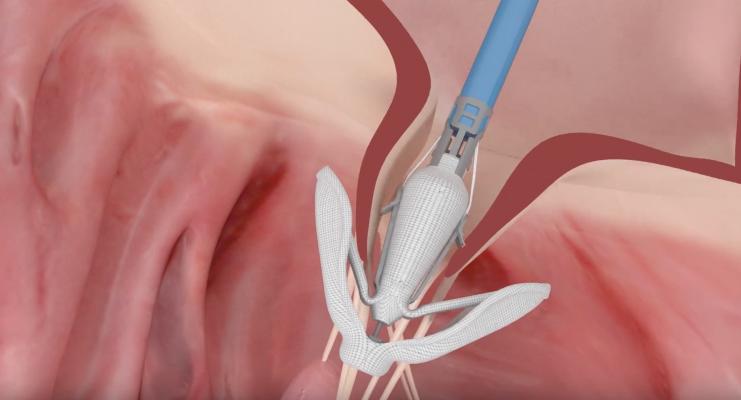
May 22, 2019 — Edwards Lifesciences Corp. announced strategic clinical and regulatory milestones for its Edwards Pascal transcatheter valve repair system at the EuroPCR annual course, May 20-23 in Paris, France. New 6-month data from the CLASP study of the Pascal system were presented by Konstantinos Spargias, M.D., from the Hygeia Hospital in Athens, Greece. In addition, the U.S. Food and Drug Administration (FDA) has approved CLASP IIF, a prospective, multicenter, randomized, controlled pivotal trial studying the Pascal system.
Patients enrolled in the CLASP study presented at EuroPCR had clinically significant mitral regurgitation (MR) despite optimal medical therapy. The results from treatment with the Pascal system demonstrated sustained positive outcomes at six months, including 81 percent of patients with mild (1+) or none/trace MR and 98 percent with <2+ MR, with echo core lab adjudication. Patients experienced clinically and statistically significant improvements in functional status, exercise capability and quality of life sustained at six months. These data build on the 30-day CLASP study results presented at the German Society of Cardiology (DGK) annual meeting in April, which also demonstrated significant reduction in MR and improvements in quality of life measures. At 30 days, the major adverse events rate was low at 6.5 percent, and there was no incidence of stroke or myocardial infarction (MI).
The FDA-approved CLASP IIF trial is designed to evaluate the safety and effectiveness of transcatheter mitral valve repair with the Edwards Pascal system compared with the Abbott MitraClip device, for the treatment of moderate-to-severe (3+) or severe (4+) functional mitral regurgitation (FMR) in symptomatic heart failure patients. The study is expected to begin enrolling in the next few months. Edwards already has underway the CLASP IID U.S. pivotal trial, which is currently enrolling patients with symptomatic primary mitral regurgitation.
The Pascal system received CE Mark and is available commercially in Europe; it is not approved in the United States.
For more information: www.edwards.com


 November 14, 2025
November 14, 2025 









