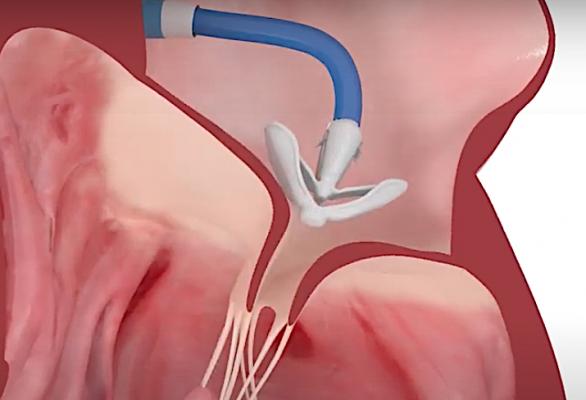
May 18, 2020 — The Edwards Lifesciences Corp. Pascal tricuspid transcatheter valve repair (TTVR) system received European CE mark for the treatment of patients with tricuspid regurgitation (TR).
"Although the prevalence of tricuspid valve disease and the associated mortality are high, there are limited effective treatment options for these very symptomatic patients, who often cannot have surgery due to the prohibitive risk," said Prof. Jörg Hausleiter, M.D., Medizinische Klinik der Ludwig-Maximilians-Universität München in Munich, Germany. "Transcatheter tricuspid therapy can be challenging due to the fragile leaflets and the large defects during valve closure. In our experience, the PASCAL system's independent grasping ability as well as the flexible and less traumatic clasp design are important features for our patients."
The Pascal system is indicated in Europe for the percutaneous reconstruction of the tricuspid valve through leaflet repair by tissue approximation. The clasps and paddles gently grasp the leaflets to facilitate coaptation, while the spacer is designed to fill the regurgitant orifice area and prevent backflow. The clasps can be operated independently to facilitate optimized leaflet capture and the implant can be elongated to a narrow profile, allowing for safe maneuvering in dense chordal anatomy.
In early clinical experience, the Pascal repair system demonstrated high procedural success and significant clinical improvements in patients with challenging tricuspid anatomy and severe TR. Sustained TR reduction was observed at 30 days, with 85 percent of patients seeing a reduction to TR ≤2+ on a five-grade scale.
Edwards continues to build a body of clinical evidence for transfemoral tricuspid therapies, including with the CLASP II TR pivotal study investigating the PASCAL system in patients with symptomatic functional or degenerative TR, the TRISCEND study investigating the EVOQUE system for tricuspid valve replacement, as well as real-world experience. The launch of the PASCAL system for the treatment of TR patients in Europe is focused on procedural success and differentiated patient outcomes, with a high-touch clinical support model.
This is the second tricuspid valve repair system to be cleared for use in Europe. The first was the Abbott TriClip device in April 2020, which is an iteration of its widely used MitraClip device. Read more in the article Transcatheter Tricuspid Valve Repair Clip Device Cleared in Europe.
The Pascal repair system is not approved in the United States. The Edwards CLASP TR EFS (CLASP TR EFS) trial in the U.S. is currently enrolling patients at numerous centers.
Prof. Jörg Hausleiter is a consultant for Edwards and a member of its Advisory Board, receiving compensation for services including proctoring, educational presentations, expert advice and R&D insights, as well as associated travel.
For more information: www.edwards.com
Related Transcatheter Tricuspid Valve Repair Content:
Transcatheter Tricuspid Valve Repair Clip Device Cleared in Europe
First Human Use of Transcatheter Duo Tricuspid Valve Announced in Ireland
VIDEO: Tricuspid Valve Imaging and Interventions Developing Hand-in-hand — Interview with Rebecca Hahn, M.D.
Advances in Transcatheter Tricuspid Valve Technologies
VIDEO: Tricuspid Device Clinical Trial Overview — Interview with Ori Ben-Yehuda, M.D.
8 Cardiovascular Technologies to Watch in 2020
TriClip Device For Tricuspid Regurgitation Effective at One Year in TRILUMINATE Study


 November 14, 2025
November 14, 2025 









