
Aria Health electrophysiologists (from left to right) Roger A. Marinchak, M.D., FACC Scott Spielman, M.D., FACC and Bradley Bacik, DO, FACC, are the first on the East Coast to utilize Epoch, the latest generation of the Stereotaxis Magnetic Navigation System, to perform robotic ablation of heart arrhythmias.
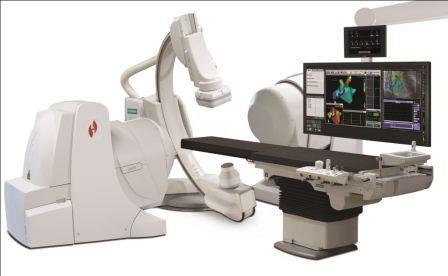
February 2, 2012 — Aria Health, the largest healthcare provider in Northeast Philadelphia and Bucks County, is the first on the East Coast to utilize Epoch, the latest generation of the Stereotaxis Magnetic Navigation System. This innovative technology allows electrophysiologists to treat many types of cardiac arrhythmias, or heart rhythm disorders, with robotic-driven catheter ablation procedures.
Housed in the heart center at Aria Health’s Torresdale Campus, Epoch offers many advantages over conventional treatment methods, according to Scott Spielman, M.D., FACC, director of Aria’s electrophysiology laboratory.
"Standard catheters used for ablation are fairly stiff, making them difficult to guide and raising the risk of perforating heart tissue,” said Spielman. “The Epoch system employs a soft, floppy catheter which enables us to complete procedures in a shorter time period with greater safety.”
The new system reduces patient radiation exposure by two-thirds, and is effective for treating ventricular arrhythmias as well as other types of abnormal heart rhythms.
“This allows us to get a catheter tip to sites inside the complex internal anatomy with less force and more precision,” said Roger Marinchak, M.D., FACC, an electrophysiologist at Aria Health. “This causes less risk of causing perforation injury to structures that one doesn’t want to ablate or hurt."
As the United States population ages, the number of people with atrial fibrillation (AF) - the most common type of serious arrhythmia - will grow, says Aria electrophysiologist Bradley Bacik, DO, FACC. While AF is a chronic condition that cannot be cured, he adds, ablation treatments available at Aria Health can substantially reduce symptoms.
For more information: www.ariahealth.org


 January 22, 2026
January 22, 2026 









