May 26, 2022 — Axon Therapies, a private company focused on addressing a root cause of heart failure, today announced positive early results from a roll-in cohort for their feasibility IDE trial, REBALANCE-HF. The study is evaluating a novel frontline therapy called Splanchnic Ablation for Volume Management (SAVM) that aims to rebalance volume distribution in heart failure patients with preserved ejection fraction (HFpEF). The results support safety and efficacy of the SAVM procedure and signaled clinical improvements in key data including pulmonary capillary wedge pressure (PCWP) with exercise and patient quality of life metrics. Data was presented at the European Society of Cardiology's Heart Failure Association 2022 annual conference in Madrid, Spain and simultaneously published online in the European Journal of Heart Failure.
"It is very promising to see the positive early dataset and improvement in key clinical outcomes. HFpEF patients have few treatment options today, and SAVM offers a minimally invasive, implant-free procedure designed to treat a root cause of their symptoms and hopefully slow down or stop disease progression – it has the potential to be a very exciting approach," commented Marat Fudim, MD, Advanced Heart Failure Specialist at Duke University Medical Center. "I'm eager to see the longer-term results and patient outcomes from the study as they become available."
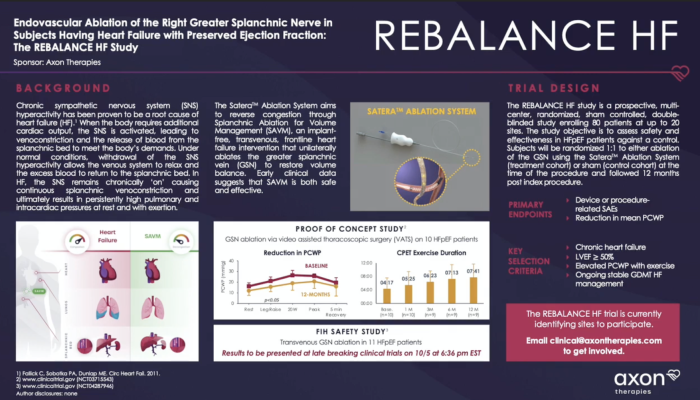
The REBALANCE-HF study is a prospective, multi-center, randomized, sham controlled, double-blinded study enrolling 80 patients at up to 20 U.S. sites and is designed to assess the safety and efficacy of the SAVM procedure. The trial included a cohort of 18 single-arm, open-label roll-in patients for interim analysis that provided early data on the SAVM procedure using the Axon Ablation Catheter. While the findings require additional confirmation, early results are very promising. The therapy had a solid safety profile—all patients were successfully treated and three non-serious device-related adverse events were reported (heart failure decompensation requiring an ER visit, transient hypertension during the procedure, and back pain following the procedure). Patients showed improvement in PCWP with exercise as well as improvements in functional capacity, symptoms, and overall health status.
Key Results
- Significant reduction in PCWP at 20 watts and peak exercise at 1-month (p=0.007 and p=0.013, respectively)
- 33% of patients experienced improvement of at least one New York Heart Association (NYHA) class compared to baseline at 1-month (p<0.01)
- Significant improvement in Kansas City Cardiomyopathy Questionnaire (KCCQ) (22.1 pts at 1-month, p <0.01; 18.3 pts at 3-months, p<0.01)
Sanjiv Shah, MD, who is the National Principal Investigator of the REBALANCE-HF trial, stated, "The SAVM procedure offers a novel approach to potentially improving outcomes in patients suffering from heart failure by reducing sympathetic nerve activity to restore volume balance within the circulation. The early data from the REBALANCE-HF trial, demonstrates the potential promise and importance of managing volume shifts in this underserved patient population." Dr. Shah is Director of Research for the Bluhm Cardiovascular Institute at Northwestern University Feinberg School of Medicine in Chicago, Illinois.
"The data for this first cohort of patients is very encouraging. We anticipate completing enrollment in REBALANCE-HF later this year and sharing the full results next year," stated Chad Hoskins, CEO of Axon Therapies. "The data builds on the results of our pilot studies, which demonstrated a durable effect of SAVM at 1 year. We're grateful for the work of our investigators, and we look forward to the culmination of these efforts in a pivotal trial beginning in 2023."
For more information: axontherapies.com


 February 03, 2026
February 03, 2026 









