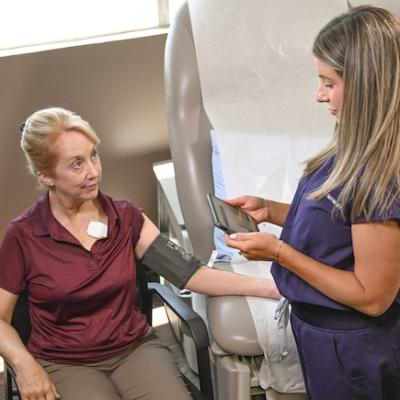
Photo: Ventric Health
Dec. 18, 2025 – Ventric Health, a medtech company enabling early detection of heart failure (HF) in a primary care setting, has announced publication of outcomes from the FDA validation study demonstrating the high accuracy of the novel Vivio System in non-invasively detecting HF using left ventricular end-diastolic pressure (LVEDP), considered the definitive HF measure. The manuscript was published online in the Journal of the American Heart Association (JAHA).
The FDA-approved Vivio System empowers earlier HF detection by enabling LVEDP measurement non-invasively in a five-minute test performed in a single primary care office visit, with the goal of earlier intervention before quality of life deteriorates.
The study included 728 patients recruited at eight U.S. sites and demonstrated that the Vivio System achieved 80% sensitivity and 83% specificity in non-invasively detecting HF using LVEDP. The system demonstrated six times greater sensitivity than echocardiography and twice the sensitivity of B-type natriuretic peptide (BNP) blood tests, two commonly used and guideline-accepted, non-invasive HF measurement methods. In the study, the Vivio System algorithm was trained with, and tested against, the most accurate LVEDP data available obtained from simultaneous recordings using an indwelling Millar Mikro-Cath catheter in the left ventricle.
The authors concluded, “The Vivio System can accurately detect elevated LVEDP and has the potential to significantly improve early detection of HF in the outpatient setting.”
They added, “Integrating the Vivio System into routine care pathways may shift HF diagnosis from reactive to proactive management, particularly for individuals with comorbidities such as diabetes mellitus or chronic kidney disease. By identifying patients before HF decompensation occurs the Vivio System may support earlier initiation of guideline-directed medical therapy (GDMT), which can improve outcomes and reduce hospitalizations.”
A recent study of more than 2,000 patients published in JACC: Advances showed that the Vivio System accurately identified previously undiagnosed HF patients who could benefit from further treatment to improve or manage their condition. Almost one-quarter of the patients identified by the Vivio System as having elevated LVEDP reported substantial symptoms and impaired health status associated with higher risks of hospitalization and death.
Additionally, a study of 1,179 patients presented at the Heart Failure Society of America Annual Scientific Meeting in September found that 71% of previously asymptomatic patients with a high risk for developing HF (Stage A HF patients) who tested positive for elevated LVEDP using the Vivio System also reported concerning symptoms on the KCCQ-12 that led to their reclassification as Stage C HF patients.
Elevated cardiac filling pressures are one of the earliest signs of HF and often precede the development of symptoms, physical findings and structural remodeling of the left ventricle. Elevated filling pressures, assessed by the direct measurement of LVEDP, is independent of the type of HF, but requires an invasive procedure and therefore is not practical to apply in the context of public health, according to the authors.
More than 24,000 patients have been tested with the Vivio System to-date. Practices using the Vivio System have reported an increase in first-time HF diagnosis in primary care, decreased hospitalization and ER visits, and improved patient medication compliance and quality of life.1
To learn more, visit www.ventrichealth.com.
References:
1. Data on file. Ventric Health.

 February 12, 2026
February 12, 2026 









