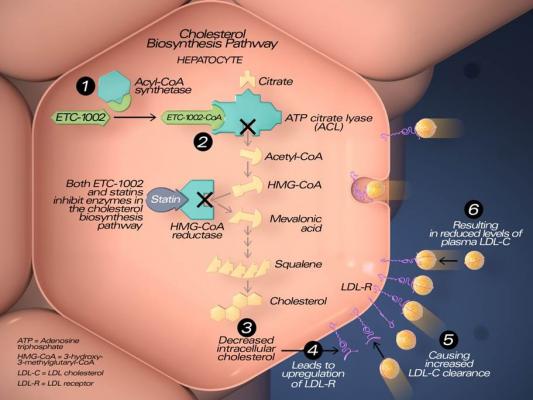
Image courtesy of Esperion
March 25, 2019 — Phase 3 results from Study 2, also known as CLEAR WISDOM, of bempedoic acid were recently presented at the American College of Cardiology (ACC) Scientific Sessions & Expo, March 16-18 in New Orleans. Bempedoic acid is being developed as a complementary, cost-effective, convenient, once-daily, oral therapy for the treatment of patients with elevated low-density lipoprotein cholesterol (LDL-C). Bempedoic acid and the bempedoic acid/ezetimibe combination tablet new drug applications have been submitted to the U.S. Food and Drug Administration (FDA), and are under regulatory review for marketing authorization by the European Medicines Agency.
The late-breaking presentation, titled “Efficacy and Safety of Bempedoic Acid Added to Maximally Tolerated Statins in Patients with Hypercholesterolemia and High Cardiovascular Risk: The CLEAR Wisdom Trial” was delivered by Anne C. Goldberg, M.D., FACP, FAHA, FNLA, professor of medicine, Division of Endocrinology, Metabolism and Lipid Research at Washington University, St. Louis.
The presentation highlighted results from the primary endpoint of LDL-C lowering at 12 weeks and key secondary endpoint of safety and tolerability over 52 weeks, including that bempedoic acid:
-
Significantly lowered LDL-cholesterol by 17 percent on background maximally tolerated statin therapy, and maintained significant reductions in LDL-cholesterol for 52 weeks;
-
Significantly lowered high-sensitivity C-reactive protein (hsCRP), an important marker of the underlying inflammation associated with cardiovascular disease, by 19 percent;
-
Had an adverse event profile that was similar to that of placebo (BA 70.1 percent vs placebo 70.8 percent) and serious adverse event profile that was generally similar to that of placebo (BA 20.3 percent vs placebo 18.7 percent);
-
Showed fewer adjudicated major adverse cardiac events compared with placebo (BA 6.1 percent vs placebo 8.2 percent);
-
Showed no worsening of glycemic measurements in patients with a history of diabetes including a 12-week reduction in hemoglobin A1c of 0.21 percent; and
-
Was observed to be safe and well-tolerated.
“In the CLEAR Wisdom Trial, bempedoic acid was shown to be safe and well-tolerated and also provided significantly lowering of LDL-cholesterol in patients on background maximally tolerated statin therapy,” said Goldberg. “These results show that bempedoic acid could be an important new, oral treatment option for high-risk patients who require additional LDL-C lowering.”
The 52-week, global, pivotal Phase 3 randomized, double-blind, placebo-controlled, multicenter CLEAR WISDOM study evaluated the efficacy and safety of bempedoic acid 180 mg/day versus placebo. The study was conducted at 86 sites in North America and Europe. A total of 779 patients were randomized 2:1 to receive bempedoic acid or placebo. The primary efficacy objective was to assess the 12-week LDL-C lowering efficacy of bempedoic acid versus placebo. Secondary objectives included evaluating the safety and tolerability of bempedoic acid versus placebo, the 24-week and 52-week LDL-C lowering efficacy of bempedoic acid versus placebo, and its effects on other risk markers after 12 weeks of treatment, including hsCRP.
Read more about late-breaking clinical trials at ACC.19
For more information: www.esperion.com


 July 31, 2024
July 31, 2024 









