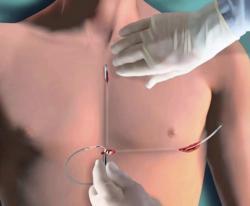
June 8, 2012 — Boston Scientific Corp. has closed its acquisition of Cameron Health, Inc. of San Clemente, Calif., and, as a result, added to its product portfolio the world's first and only commercially available subcutaneous implantable cardioverter defibrillator, called the S-ICD System. The acquisition is the capstone of a nearly 10-year relationship between the two companies during which Boston Scientific invested in Cameron Health during its groundbreaking research and product commercialization efforts. Developed by Cameron Health, the entire S-ICD System sits just below the skin. This leaves the heart and blood vessels untouched, offering patients an alternative to conventional transvenous ICDs, which require thin, insulated wires – known as leads – to be placed into the heart itself.
"We believe that the combination of Cameron Health's breakthrough technology and Boston Scientific's already strong arrhythmia management product portfolio and commercial capabilities will help unlock the enormous potential of the S-ICD System," said Kevin Hykes, former CEO of Cameron Health, who will continue to lead the S-ICD team at Boston Scientific.
At the recent Heart Rhythm Society's 33rd Annual Scientific Sessions in Boston, Cameron Health announced initial results from the international Evaluation oF Factors Impacting Clinical Outcome and Cost Effectiveness (EFFORTLESS) Subcutaneous Implantable Defibrillator Registry study that showed the S-ICD System is performing appropriately in real-world circumstances and continues to demonstrate positive results in a study of 230 patients. Cameron Health also announced at the conference that the S-ICD System met the primary safety and efficacy endpoints defined in their 330-patient investigational device exemption (IDE) clinical study. The patient population in the IDE study patient population closely mirrored real world populations with transvenous ICDs, demonstrating that the S-ICD System is an important new treatment option for a wide range of primary and secondary prevention patients. In addition, the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel recommended approval of the S-ICD System in April of 2012. FDA approval is expected in the first half of 2013. The S-ICD System received CE mark in 2009 and is commercially available in many countries in Europe, as well as New Zealand. To date, more than 1,300 devices have been implanted in patients around the world.
In countries where it is approved, the S-ICD System establishes a new class of protection from sudden cardiac arrest that offers physicians more options in how to best treat their patients. While it provides the same defibrillation protection of transvenous ICDs, the S-ICD System also preserves the patient's venous system, which may be advantageous to many patients.
Transaction Details
The transaction follows Boston Scientific's exercise of its option to acquire Cameron Health, announced March 8, 2012. Under the terms of the agreement, Boston Scientific paid $150 million at closing. The agreement calls for an additional potential payment of $150 million to be made upon FDA approval of the S-ICD System and up to an additional $1 billion of potential payments to be made upon the achievement of specified revenue-based criteria over a six-year period following FDA approval. The company currently expects the transaction to be approximately $0.01 dilutive in 2012 and approximately break-even in 2013 to earnings per share on an adjusted basis and more dilutive in both years on a GAAP basis as a result of acquisition-related net charges and amortization, which will be determined following the closing.
The S-ICD System is an investigational device and limited under U.S. law to investigational use only, and is not available for sale in the United States.
For more information: www.bostonscientific.com/cameronhealth


 January 13, 2026
January 13, 2026 









