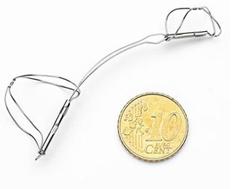
October 3, 2011 – Cardiac Dimensions Inc. announced it received European CE mark approval for a newly enhanced version of its Carillon Mitral Contour System, a therapy for treating heart failure patients suffering from functional mitral regurgitation (FMR). Receipt of the CE mark enables Cardiac Dimensions to initiate a commercial launch of the enhanced device in Europe, which will begin in 2012.
The CE mark approval follows the successful completion of the AMADEUS and TITAN clinical trials of the Carillon system, which demonstrated significant reduction in FMR and significant improvement in functional capacity and quality of life. An additional trial with the enhanced version of Carollon, called TITAN II, was recently initiated to further the clinical experience.
Janusz Lipiecki, M.D., of Clinique Pole Sante Republique in Clermont Ferrand, France, one of the TITAN and TITAN II investigators, commented, "FMR secondary to left ventricular dilatation is associated with poor prognosis and high rates of mortality. As a new treatment option, Carillon offers compelling and unique attributes given its rapid percutaneous deployment and innovative use of natural cardiac structures to reduce mitral annulus dimension. The elegance and ingenuity behind the design allow for customization based on patient anatomy as well as intra-operative assessment to ensure safety. From a clinical perspective, patients have experienced early and sustained improvements that suggest profound utility. I am pleased to hear of the recent CE mark approval and excited to serve as a TITAN II investigator and further contribute to the clinical experience."
The mitral contour system combines a proprietary, implantable device with a percutaneous catheter delivery system. The implantable device consists of a proximal anchor and a distal anchor connected by a shaping ribbon. Utilizing the heart's natural structures, the device is intended to reduce mitral annulus dilatation upon deployment, thereby significantly reducing FMR. Rapidly delivered via the venous vasculature, Carillon has the potential to treat most heart failure patients in a minimally invasive fashion. Early clinical data suggest that usage is associated with significant reduction in FMR, and significant improvement in functional capacity and quality of life.
For more information: www.cardiacdimensions.com


 December 24, 2025
December 24, 2025 









