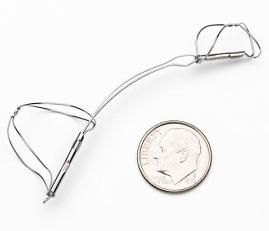
February 2, 2009 - Cardiac Dimensions Inc. today said it received the CE Mark approval for its CARILLON Mitral Contour System from KEMA Quality B.V., a European Union Notified Body from the Netherlands.
The CARILLON system is a percutaneous treatment for functional mitral regurgitation (FMR). Cardiac Dimensions is now developing plans for commercialization in Europe. The company plans to work with the FDA for a final pivotal trial in the U.S.
About 5 million people in the U.S. and more than 20 million people worldwide suffer from heart failure. Most of these patients also suffer from dilated cardiomyopathy and functional mitral regurgitation (FMR); the majority of whom are inadequately treated using medical management. While surgical options exist and can be effective in reducing FMR, they are infrequently used due to the burden of the surgery itself, which can be associated with high operative morbidity and mortality rates.
The CARILLON Mitral Contour System combines a proprietary implantable device and delivery system. The implant consists of a shaping ribbon between distal and proximal anchors. It is delivered percutaneously via jugular vein access under fluoroscopic guidance. The implant is designed to be positioned, adjusted and gently anchored in the coronary sinus/great cardiac vein to reshape the annulus around the mitral valve, thereby reducing mitral regurgitation. Preclinical and early clinical data have suggested both a reduction in mitral regurgitation and improvements in other key parameters including NYHA class, six minute walk distances and quality of life, the company said.
For more information: www.cardiacdimensions.com


 December 24, 2025
December 24, 2025 









