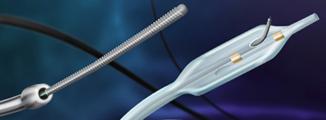
The CrossBoss and Stingray CTO catheters.
November 24, 2009 – European CE mark clearance was granted this week for BridgePoint Medical’s coronary and peripheral chronic total occlusion (CTO) crossing system comprised of the CrossBoss CTO Crossing Catheter and the Stingray CTO Re-Entry System.
The National Heart, Lung and Blood Institute (NHLBI) estimates CTOs are found in about one-third of patients who undergo angiography. Interventional cardiologists are currently unable to broadly treat patients with CTOs due to the procedurally challenging and advanced nature of the disease. For these patients, alternatives include bypass surgery or pharmacologic therapy.
“We have seen many attempts to implement new tools for the treatment of chronic coronary occlusions over the years, but until now none of them have provided any advantage over the current treatment of using specialty guide wires,” said Gerald Werner, M.D., Ph.D., professor at the Klinikum Darmstadt in Darmstadt, Germany. “This system is the first to provide a new option for the frequent situation when our guide wires do not reach the vessel beyond the occlusion. The BridgePoint system makes it possible to find the true vessel lumen and conclude the treatment successfully.”
The company said its disposable catheter system does not require the use of expensive and cumbersome capital equipment and uses techniques that are common in interventional medicine.
The CrossBoss and Stingray are indicated in the European Union for adults with coronary or peripheral artery disease, age 18 and older. The devices are contraindicated for use in cerebral vasculature.
In a prospective clinical trial conducted in the European Union and a post-market registry conducted primarily in South America, the BridgePoint System was 67-85 percent successful in placing an interventional guide wire beyond a chronic total coronary occlusion. Complications with the system were similar to general PCI and stenting.
The BridgePoint devices are currently under clinical investigation in the United States and are not commercially available for treating CTOs.
For more information: www.bridgepointmedical.com


 October 28, 2025
October 28, 2025 









