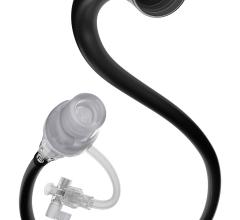
May 10, 2022 — The MIVI Neuroscience Q Aspiration Catheter incorporates a novel pusher wire design on its proximal end. This feature has demonstrated in simulated use studies that the Q Catheter may be uniquely suited to allow physicians in the future to treat ischemic stroke patients remotely via a robot from a different room, a different city, or even a different country.
Dr. Demetrius Lopes of Advocate Lutheran General Hospital in Park Ridge, Illinois, has conducted flow-model compatibility testing with the Q catheter and the Corindus GRX robot. Using a simulated neurovascular model, Lopes has been able to show preliminary compatibility between the Q Catheter and the robot system and perform a successful thrombectomy procedure. Lopes will present his experience at the World Live Neurovascular Conference (WLNC) May 11-13 in Washington, D.C.
The Corindus GRX Vascular Robotic system is currently approved for use in endovascular coronary and peripheral therapies, and FDA approval for a neurovascular indication may be on the horizon. The robotic system is designed to be used with rapid exchange angioplasty balloons and balloon-mounted stents, which is the preferred platform in cardiology.
Due to its unique design, the Q Catheter functions with the robot very similarly to these rapid exchange devices. The Q Catheter, currently FDA cleared for use with compatible guide catheters in facilitating the insertion and guidance of microcatheters into a selected blood vessel in the peripheral, coronary and neuro vascular systems, is not currently cleared for thrombectomy but is currently in a clinical study for future market clearance in the U.S.
“Not only does the Q Catheter’s design maximize aspiration force for the best chance at revascularization, it also overcomes the challenge of the robotic system and limitations of traditional catheters” said Lopes. “I was very pleased with the Q Catheter tracking and aspiration performance in this initial testing with the robot.”
Bob Colloton, CEO of MIVI Neuroscience, commented “As a company, our goal is to meet the unmet needs of our neurovascular physicians with innovative solutions. Regardless of where or how the procedure is performed, we want physicians to be able to choose the Q Catheter because it offers the highest aspiration power and a simple, single-operator setup. We are proud to partner with Dr. Lopes to show that the Q Catheter is well-poised for the future of mechanical thrombectomy.”
Q Catheter for Neurovascular Thrombectomy: CAUTION Investigational device. Limited by United States law to investigational use.
For more information: www.mivineuro.com
Related Robots in the Cath Lab Content:
VIDEO: Robotic PCI Performed Well in Real-World Population in the PRECISION GRX Study — Ehtisham Mahmud, M.D.
Second Generation Robotic PCI System Performs Well Across Spectrum of Lesion Complexity
VIDEO: Standardizing PCI Through Smart Robotic Procedural Automation
FDA Clears Corindus CorPath GRX for Peripheral Vascular Interventions
VIDEO: Corindus CorPath Robotic PCI System For The Cardiac Cath Lab
Corindus CorPath Used in World's First-in-Human Telerobotic Coronary Intervention
14 Ways to Reduce Radiation Exposure in the Cath Lab
Corindus Seeking Neurovascular Intervention Clearance for CorPath GRX Vascular Robotic System
Innovations Driving the Cath Lab Technology of Tomorrow
Siemens Completes Acquisition of Cath Lab Robotics Vendor Corindus
First Robotic Coronary Angioplasties Performed With Robocath System in Germany
Hoag Performs First Robotic Carotid Artery Stenting on West Coast


 January 19, 2026
January 19, 2026