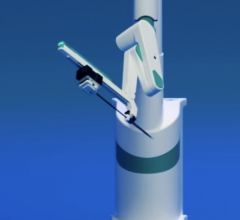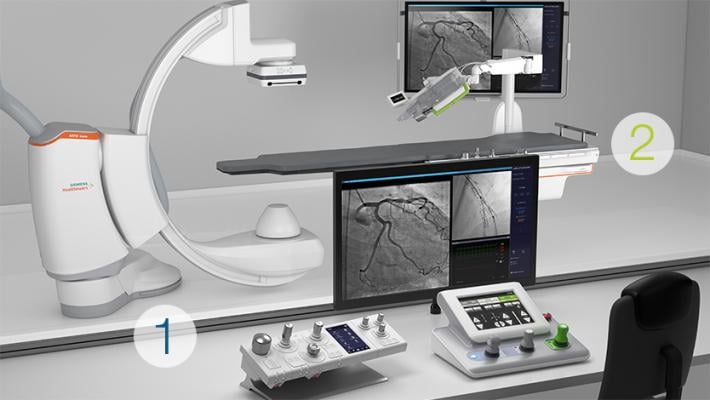
Corpath GRX interventional cardiology robotic system is comprised of two different components: 1. The control console. 2. The bedside unit.
December 16, 2020 — Corindus, A Siemens Healthineers company and a leading developer of vascular robotics, began its global launch of a new set of automated robotic movements in the technIQ Series designed for the CorPath GRX System. The company received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for new software automation that provides predictable and consistent movements to aid in advanced device manipulation during complex coronary and peripheral procedures.
Jean Fajadet, M.D., the co-director of interventional cardiology at Clinique Pasteur in Toulouse, France, performed the first in-human coronary procedures with the benefit of the new automated movements, which replicate manual techniques of highly skilled interventionalists. The procedures demonstrated how the new movements can help to reduce procedure time associated with wire and device manipulation and drive standardization in quality of care by offering advanced techniques to all physicians.
“This new software algorithm gives the operator new possibilities to advance and facilitate treatment, especially with complex lesions,” Fajadet said. “I am pleased with the success of using the automated moves and am honored to play a role in the advancement of robotic technology that will make interventional procedures more efficient and safer for our patients.”
In 2018, Corindus received CE mark and FDA clearance for an automated technique called Rotate on Retract (RoR). RoR was the first automated robotic movement in the technIQ Series and has demonstrated the potential to significantly reduce wiring time. The latest global introduction and FDA clearance for the technIQ Series provides physicians with four additional automated robotic movements that aid with complex tasks such as crossing lesions, navigating tortuosity, and precisely measuring the anatomy for appropriate device size selection.
“The new technIQ movements mark the next phase in the evolution of robotic-assisted intervention and a vital step toward the advancement of our technology,” said Wayne Markowitz, Executive Vice President and Head of Corindus. “Automating more movements used in cardiovascular intervention will allow physicians to focus their attention on overall case strategy while equipping them with advanced techniques for navigating the vasculature.”
For more information: www.corindus.com
Related Cardiology Robotic System Content:
VIDEO: Standardizing PCI Through Smart Robotic Procedural Automation
Final Results of the Multicenter PRECISION GRX Study of the CorPath in a real-world population across a spectrum of lesion complexity — SCAI 2021 late-breaker
Robocath Successfully Carries Out First Robotic Coronary Angioplasties in Humans
Corindus Vascular Robotics to Be Acquired by Siemens Healthineers
VIDEO: Corindus CorPath Robotic PCI System For The Cardiac Cath Lab
Reducing Physician Radiation Dose With Robotics
Corindus Seeking Neurovascular Intervention Clearance for CorPath GRX Vascular Robotic System
Corindus CorPath Used in World's First-in-Human Telerobotic Coronary Intervention
Hoag Performs First Robotic Carotid Artery Stenting on West Coast
Find more information on robotic systems for the cath and EP labs


 January 27, 2026
January 27, 2026