September 15, 2014 — Claret Medical Inc. announced that it has entered into an agreement for up to $18 million in a Series B financing. The Series B round was led by Santé Ventures, a prominent healthcare-focused venture capital firm with $260 million in capital under management, with participation from Lightstone Ventures, a leading venture capital firm with $172 million under management. Easton Capital also joined the round. James Eadie from Santé Ventures and Hank Plain from Lightstone Ventures will be joining the company’s board of directors.
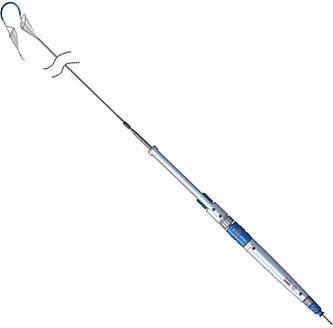
Proceeds will be used primarily to complete the U.S. pivotal trial of the company’s Sentinel Cerebral Protection System (CPS) for embolic protection during transcatheter aortic valve replacement (TAVR), which received Investigational Device Exemption (IDE) approval earlier this year.
The Sentinel CPS is the only filter-based system on the market that both captures and removes embolic debris released during TAVR procedures that could otherwise be a source of peri-procedural stroke. The system has been used in more than 800 procedures worldwide to date.
“Many consider the stroke rate in TAVR concerning, especially as clinicians look to bring the benefits of TAVR to lower risk and younger patients,” said Azin Parhizgar, Ph.D., president and CEO of Claret Medical. “The proceeds of this financing round will enable Claret Medical to conduct the SENTINEL pivotal trial, which we expect to confirm the therapeutic importance of embolic debris capture and removal during TAVR. This same approach has long been established in carotid stenting procedures.”
“The SENTINEL trial will evaluate the role of the Sentinel CPS in reducing the number and size of new ischemic lesions in the brain and their potential impact on patients’ neurocognitive function,” said Martin Leon, M.D., director of the Center for Interventional Vascular Therapy at Columbia University Medical Center in New York and chair of the trial’s Clinical Steering Committee. “By doing so, the trial will help define the field of cerebral protection during TAVR in order to pave the way for safer TAVR procedures.”
The company is also embarking upon two other studies of structural heart procedures that could benefit from the use of the Sentinel CPS in reducing cerebrovascular events during complex surgical and transcatheter procedures.
For more information: www.claretmedical.com


 December 24, 2025
December 24, 2025 









