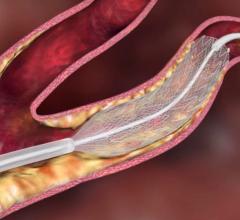
Feb. 6, 2007 — The Centers for Medicare and Medicaid Services (CMS) has released new proposed policy in which coverage for carotid artery stenting (CAS) would be expanded. The National Coverage Determinaton (NCD) would encompass an asymptomatic patient population (approximately 70 percent of patients).
Under the proposed policy, asymptomatic patients under age 80 who are at high risk for surgery and have 80 percent or greater carotid artery stenosis would be eligible for coverage outside of a post-market study or clinical trial as long as the procedure is performed using FDA-approved Carotid Artery Stent (CAS) systems and embolic protection devices in a facility approved by CMS. Patients over age 80, with 50-70 percent stenosis would still have to be enrolled in a post-market trial in order to receive coverage.
The carotid stent market is approximately $100 million in the U.S., according to a statement from Abbott Vascular. The impact of the CMS policy decision represents a significant, positive impact — 50-75 percent market growth or $50-75 million on an annual basis.
Boston Scientific released a statement indicating it supports the proposed expansion but also supports broader coverage.
“We are excited about the opportunity for expanded coverage for carotid stenting for asymptomatic patients at high risk for surgery,” said John Pedersen, president of Boston Scientific’s Peripheral Interventions business. “However, we believe that clinical data from our carotid stenting trials also supports expansion of coverage for high-risk symptomatic patients.
“This proposal comes on the heels of Boston Scientific’s launch of the NexStent Carotid Stent and the FilterWire EZ Embolic Protection System, two products that utilize state-of-the art technology to address the need for stroke protection in high surgical risk patients with carotid artery stenosis,” added Pedersen. “We look forward to continuing to provide CMS with data to support even broader coverage of carotid stenting.”
Currently, Medicare only provides coverage for CAS to a small subset of high-risk Medicare beneficiaries who are symptomatic and have 70 percent or greater carotid artery stenosis. Under the proposed policy, other high-risk and non–high risk Medicare patients, including octogenarians, will be required to enroll in a category B investigational device exemption (IDE) clinical trial or an FDA-mandated post-approval study to access coverage for CAS.
The proposed coverage change, says Boston Scientific, reflects the medical community’s growing acceptance of the procedure as a viable alternative to surgery for high-risk patients with carotid artery disease.
There will be a public comment period, with final decision expected on May 3, 2007 — if approved, coverage could be effective that same month.
For more information visit www.boston.scientific.com.

 June 26, 2023
June 26, 2023