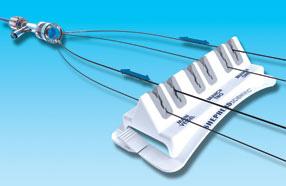
September 21, 2010 – Two companies announced that they have entered into a distribution agreement in the United States. The agreement allows Vascular Solutions to distribute Shepherd Scientific’s Angio Assist and Teirstein Edge products.
The Angio Assist is a docking station that facilitates the introduction of guide wires into interventional devices such as catheters and atherectomy burrs. The Teirstein Edge is designed to organize guidewires and catheters during interventional procedures. Both products have received 510(k) clearance with the U.S. Food and Drug Administration (FDA) for sales in the United States and will be sold and serviced through Vascular Solutions’ direct sales force.
The new products will be featured at the Transcatheter Cardiovascular Therapeutics (TCT) conference inWashington, D.C. later this week.
For more information, please visit: www.shepherdscientific.com or www.vascularsolutions.com


 October 28, 2025
October 28, 2025 









