January 28, 2014 — The Gagnon Cardiovascular Institute at Morristown Medical Center adopted a minimally invasive medical device to treat patients with severe aortic stenosis who are too ill or frail to have their aortic valves replaced trough traditional open-heart surgery.
The U.S. Food and Drug Administration (FDA) approved the CoreValve System after a
clinical study. The Extreme Risk Study of the CoreValve U.S. Pivotal Trial involved 45 U.S. sites, including Morristown, N.J., and demonstrated low rates of common complications such as stroke and valve leakage.
A 79-year-old male with symptomatic severe aortic stenosis who was too ill to undergo traditional aortic
valve replacement became third in the nation to receive a commercial CoreValve procedure at Morristown.
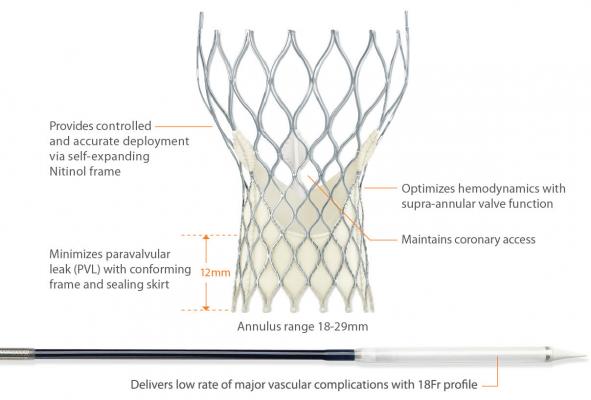
Most commonly, the CoreValve System is inserted into a patient via an artery in the leg and the physician guides it through the arteries into the heart at the site of the original aortic valve. Once in place, it takes over the original valve's function to ensure blood flows out of the heart efficiently and circulates throughout the body.
The design of the new device was developed to address needs of the transcatheter aortic valve replacement (TAVR) patient population, serving a broad spectrum of severe aortic stenosis patients. The CoreValve System is suitable for patients with native valves of nearly all sizes, and is delivered via a small delivery system, making it possible to treat patients with vascular systems that are small or difficult to navigate. Additionally, the CoreValve System enables physicians to deliver the device to the diseased valve in a controlled manner, allowing for accurate placement.
For more information: www.corevalve.com