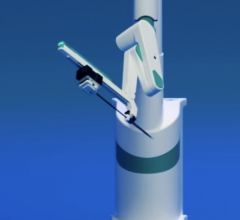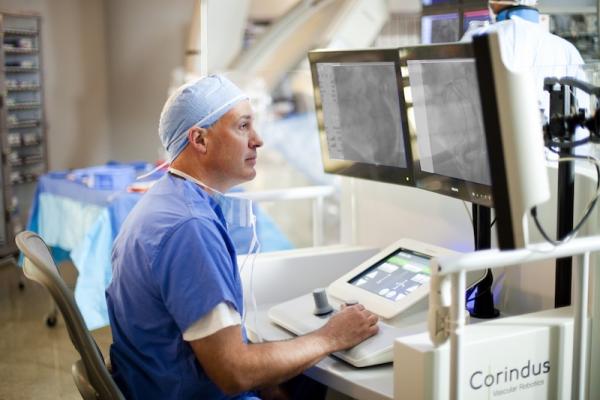
June 7, 2014 — Corindus Vascular Robotics has been named the recipient of the 2014 North American New Product Innovation Award in Interventional Cardiology, which was established by Frost & Sullivan to recognize visionary innovation and product excellence. Corindus’s CorPath vascular robotic system is the first medical device to bring robotic precision and accuracy to coronary angioplasty procedures, which may improve clinical outcomes while increasing radiation protection for interventional cardiologists.
“To achieve excellence in new product innovation leadership is not an easy task, but the CorPath system addresses a critical concern of interventional cardiologists, and has the potential to improve outcomes and economics in coronary angioplasty,” said Greg Caressi, senior vice president, Healthcare & Life Sciences, Frost & Sullivan. “Corindus’s receipt of this award signifies a great accomplishment.”
The CorPath System stood out from other products in the market with differentiators such as:
- Fulfilling Unmet Needs in Interventional Cardiology. The CorPath system provides tremendous benefits that decrease key career-limiting issues that physicians tend to face as a result of radiation exposure.
- Optimized Design Considerations for Cath Lab Use. The system was designed to have the physician sitting in the interventional cockpit, removed from radiation, in a robotic control unit that sits beside the patient on the patient table bedrail.
- Advancing Care and Safety. It can safely navigate through the vascular anatomy to perform balloon stenting techniques.
- Innovative CorPath One Stent Program. Corindus Vascular Robotics launched the “CorPath One Stent Program” to highlight the precision and accuracy of the system by offering a $1,000 credit to hospitals that use two or more stents per lesion in coronary angioplasties performed with CorPath.
- Future Promise. The CorPath system is believed to have a future in emerging interventional techniques and could be leveraged for procedures in other related fields.
For more information: www.frost.com, www.corindus.com


 January 27, 2026
January 27, 2026